Volume 7, Issue 1 (Continuously Updated 2024)
Func Disabil J 2024, 7(1): 0-0 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Rafati S, Kamali M, Nabovati P. Vision Rehabilitation Using Contact Lenses in Infantile Nystagmus: A Systematic Review. Func Disabil J 2024; 7 (1) : 205.1
URL: http://fdj.iums.ac.ir/article-1-247-en.html
URL: http://fdj.iums.ac.ir/article-1-247-en.html
1- Department of Optometry, Rehabilitation Research Center, School of Rehabilitation Sciences, Iran University of Medical Sciences, Tehran, Iran.
2- Department of Rehabilitation Management, Rehabilitation Research Center, School of Rehabilitation Sciences, Iran University of Medical Sciences, Tehran, Iran.
3- Department of Optometry, Rehabilitation Research Center, School of Rehabilitation Sciences, Iran University of Medical Sciences, Tehran, Iran. ,nabovatipayam@yahoo.com
2- Department of Rehabilitation Management, Rehabilitation Research Center, School of Rehabilitation Sciences, Iran University of Medical Sciences, Tehran, Iran.
3- Department of Optometry, Rehabilitation Research Center, School of Rehabilitation Sciences, Iran University of Medical Sciences, Tehran, Iran. ,
Keywords: Vision rehabilitation, Contact lenses (CLs), Infantile nystagmus (IN), Systematic review, Congenital nystagmus, Rigid gas permeable lenses, Quality of life (QoL)
Full-Text [PDF 1208 kb]
(603 Downloads)
| Abstract (HTML) (1096 Views)
Full-Text: (314 Views)
Introduction
Infantile nystagmus (IN) is an involuntary rhythmic oscillation of the eyes that usually occurs in the first 3 to 6 months of life [1] and its prevalence in the general population is reported to be 0.14% [2]. This disorder is classically divided into afferent nystagmus (sensory defect) and efferent nystagmus (idiopathic). Afferent nystagmus mainly occurs in the first few months of life due to insufficient image formation in the fovea, which is caused by anatomical changes or organic defects in the eye [3]. Among the most common causes of afferent nystagmus are eye diseases, such as congenital cataracts, albinism, and corneal opacity, as well as developmental abnormalities of the optic disc and retina such as Leber’s congenital amaurosis, achromatopsia or congenital stationary night blindness [4]. Efferent or idiopathic nystagmus, on the other hand, is a motor functional disorder that occurs without any ocular or systemic pathology [5].
Nystagmus, with a lifelong negative effect on the quality of life (QoL) of patients, leads to an increase in their dependence on others and a decrease in self-confidence and social relationships [6]. Effective management and treatment are crucial for enhancing visual function and social engagement. The objectives of nystagmus treatment include optimizing vision by increasing foveation periods, reducing nystagmus severity, correcting abnormal head positions, and addressing strabismus [7]. Non-surgical management strategies for nystagmus include medications, biofeedback techniques, acupuncture, prismatic glasses, and contact lenses (CLs). This article focuses on assessing the impact of CLs on this disorder.
Research has evaluated the effectiveness and performance of both soft and rigid gas permeable (RGP) CLs on nystagmus, noting that each type may improve visual performance due to its distinct characteristics.
The first goal of this study was to systematically review the literature for evidence that shows the effect of using CLs on the visual performance of a person with congenital nystagmus and then to find the differences between soft and hard CLs. Our secondary goal was to create a framework to better understand the advantages and disadvantages of using CLs in people with congenital nystagmus for the clinical management of patients with IN. As a result, this review article examined the evidence available in the medical literature in response to these questions.
Methods
At first, we looked for studies on the effect of hard and soft CLs on congenital nystagmus patients by reviewing the literature.
The proposed systematic review will answer the following question:
Do IN patients who wear hard CLs have better vision than those who wear soft CLs?
Search strategy
This systematic review was conducted according to the guidelines set forth in the statement of preferred reporting items for systematic reviews and meta-analyses (PRISMA). We searched PubMed, Scopus, Google Scholar, and the Cochrane Central Register of Controlled Trials for relevant studies from inception to May 9, 2022. We excluded review articles and imposed no restrictions on publication time or language. The search utilized medical subject headings (MeSH) and keyword combinations of nystagmus, contact lens, congenital nystagmus, IN, rigid gas permeable lenses, and soft CLs. Boolean operators “AND” and “OR” were used to refine the search.
Eligibility criteria
Inclusion criteria
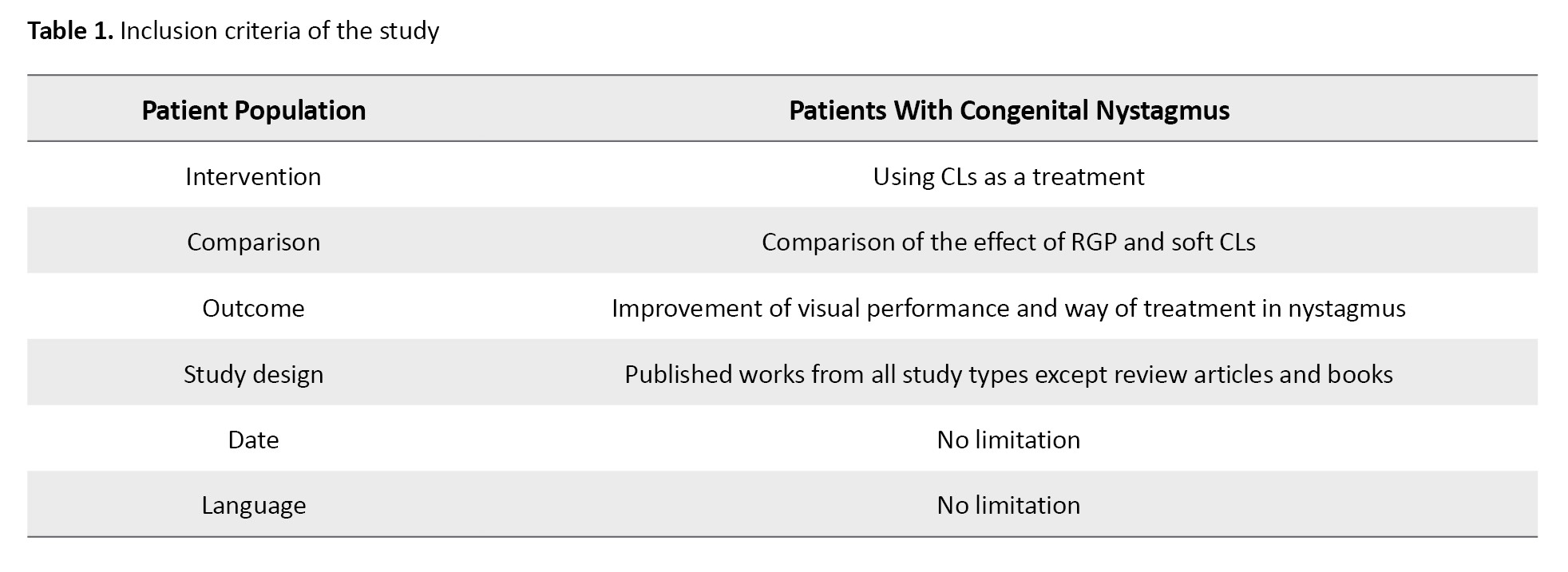
Inclusion criteria were formulated using the participants, intervention, comparison, outcomes, and study designs (PICOS) strategy. Studies were selected based on the following criteria: 1) Type of study: All types of studies available based on the PRISMA criteria in the field of IN, 2) Participants: All individuals with neonatal nystagmus, 3) Intervention: The use of soft or hard CLs in patients with congenital nystagmus, 4) Outcomes: Improvement of visual functions, including visual acuity, contrast sensitivity, and reading performance, as well as improvements in the intensity, range, and frequency of eye movements. The inclusion criteria are given in Table 1.
Exclusion criteria
Given the scarcity of studies in this area, the exclusion criteria were minimal, excluding only animal studies and studies involving patients who underwent various types of surgery prior to the intervention.
Quality assessment
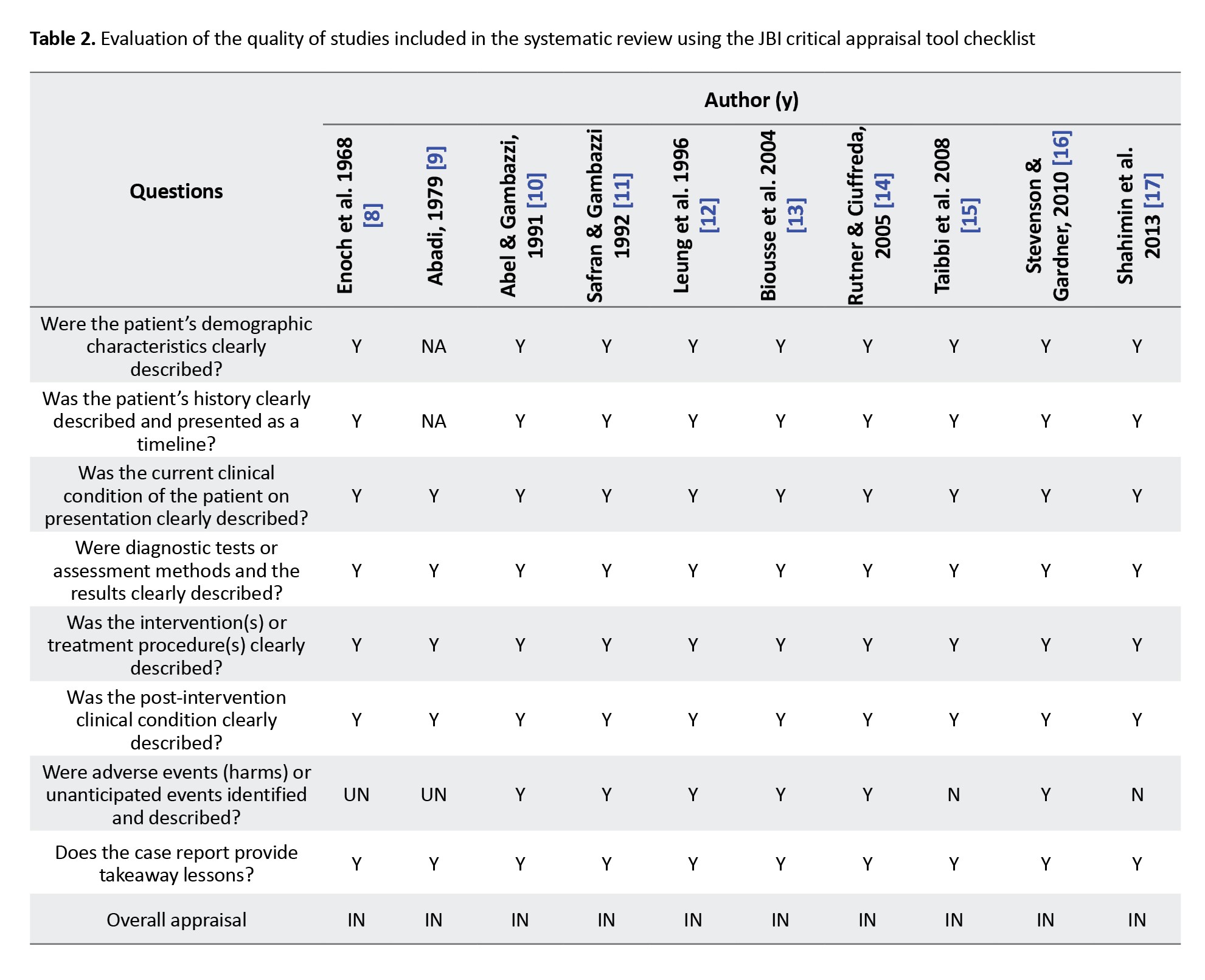
The quality of the studies was evaluated using the JBI critical appraisal tool checklist for case series and case reports (Table 2).
Factors evaluated for potential bias included clarity and impartiality of inclusion criteria, the objectivity of exposure assessment (the condition measured), the employment of valid methods, comprehensive reporting of demographic profiles, completeness of case information, and the appropriateness of the statistical analyses used.
Results
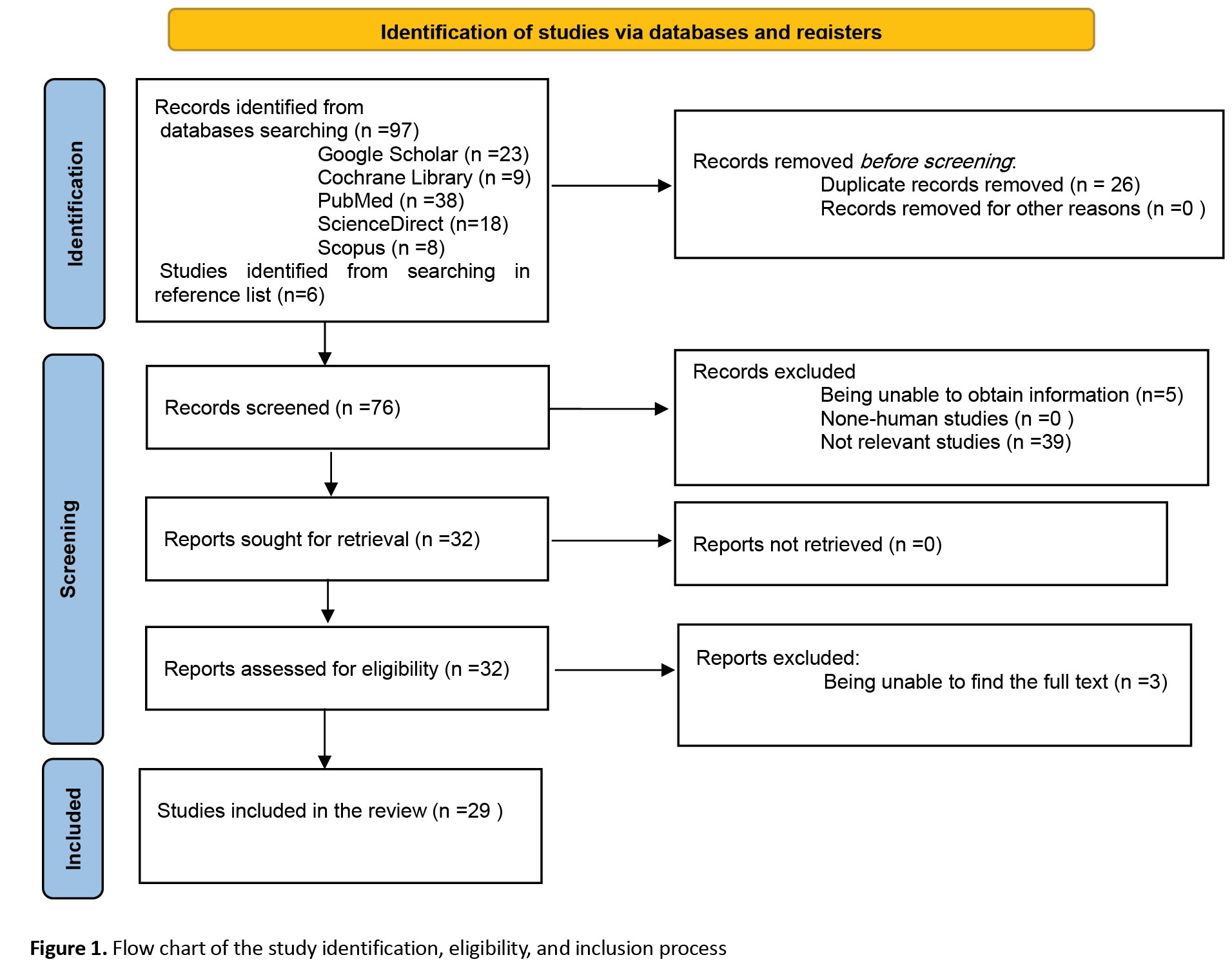
A total of 38 articles were identified in PubMed using MeSH keywords, and 8 articles were found in Scopus review. From the Cochrane Central Register of Controlled Trials (CENTRAL), 9 articles were identified, with 2 overlapping with those found in Scopus. In Google Scholar, after utilizing advanced search criteria on titles and abstracts, 23 articles were identified, and an additional 6 articles were found. A list of other related articles was compiled through reference checking.
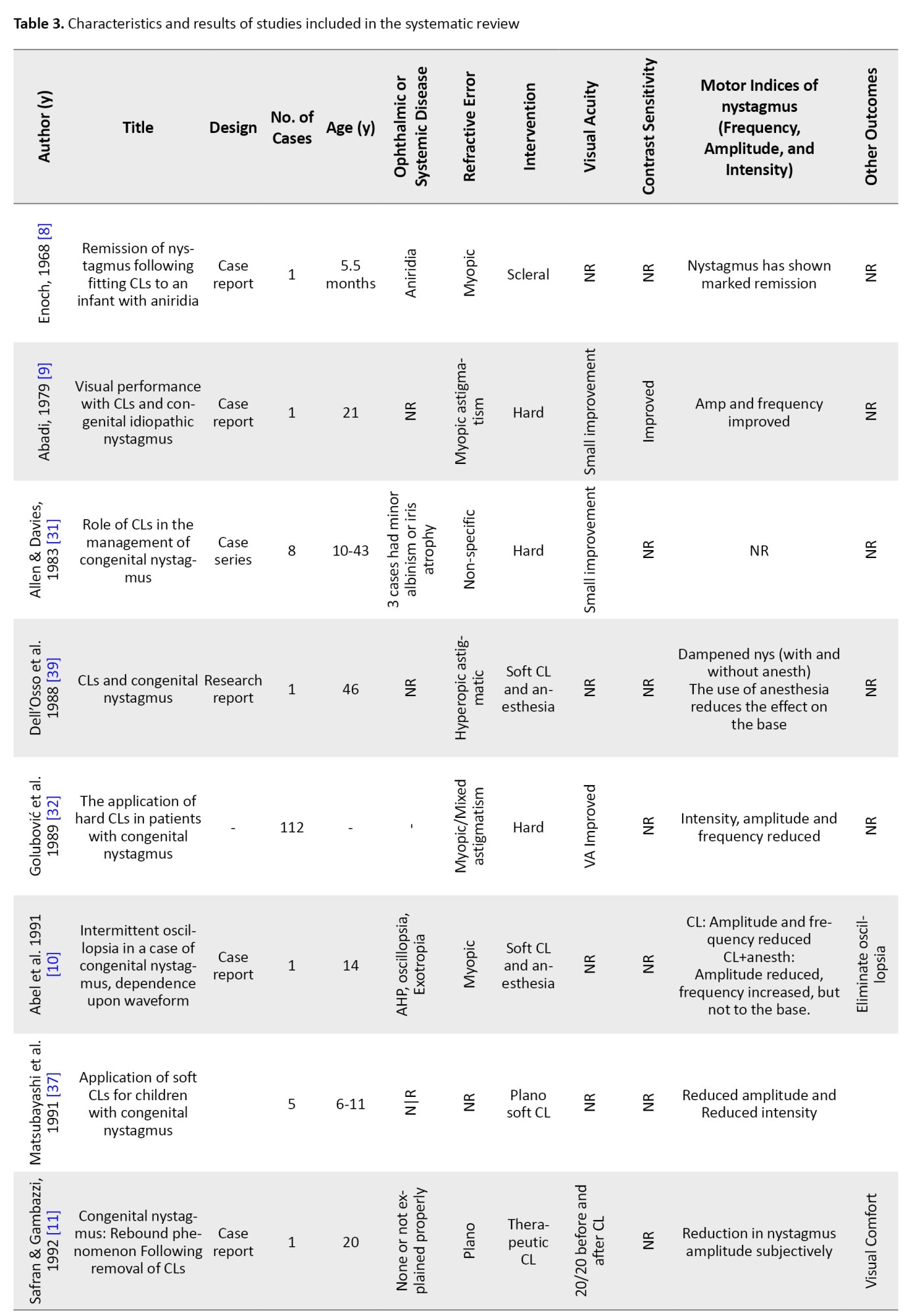
Initially, out of 102 articles found, after removing duplicate articles, 76 articles remained, and then in the screening, 39 articles were removed from the remaining 76 articles due to being unrelated and 8 articles due to the lack of access to the abstract or full text. A total of 29 articles were included in this systematic review. The process of identifying studies is shown in Figure 1. Table 3 shows the characteristics and results of the studies.


Data extraction
After completing the database search and removing duplicates by title check, titles, and abstracts were screened to identify potentially eligible articles. Full texts of these articles were then reviewed to ensure they met the inclusion and exclusion criteria. For the included studies, data were extracted on study characteristics (authors, year, study design, follow-up time, interventions, and controls), patient characteristics (age, changes in visual acuity, changes in intensity, frequency of nystagmus, and contrast sensitivity).
Discussion
The application of CLs in patients with congenital nystagmus has been documented in scientific literature since 1962 [18], yet research in this area remains sparse, and the few existing studies have yielded conflicting results.
There is only one RCT study in this field. Aside from the one RCT in this field, the vast majority of research consists of case reports with small sample sizes. Additionally, these studies generally involve short-term follow-ups and overall, do not possess strong methodologies. As a result, we decided to have a complete review of the articles in this field. Initially, it is essential to determine which mechanisms most likely contribute to the effects of CLs on IN.
Refractive errors and nystagmus
Considering the higher prevalence of refractive errors in people with IN compared to the general population [19] and the wide range of refractive errors found in these patients, correcting refractive errors in these people is important. Even minimal corrections can improve visual perception. The incidence of corneal astigmatism is notably high in individuals with IN [20-22], with 57% experiencing astigmatism greater than 2.00 diopters [21]. Studies suggest that astigmatism increases with age [23] and is likely caused by continuous interactions between the cornea and eyelid during eye movements, which predominantly occur along the horizontal axis, although vertical or torsional movements may also be present [22, 24]. There is no consensus about the prevalence of myopia [21] and hyperopia [25] in different studies.
The initial step in managing IN involves correcting refractive errors, typically through glasses or CLs. For high refractive errors, glasses pose challenges due to constant eye movement, preventing consistent focus through the optical center of the glasses [26]. As a result, when patients look through areas other than the optical center, they experience prismatic effects, spherical, and chromatic aberrations, which interfere with the foveation period and impair sensory system function. In contrast, CLs move with the eyes, ensuring optimal visual acuity through the optical center at all times. They minimize adverse effects associated with glasses, such as magnification and minification, particularly in cases of high refractive errors, and reduce prismatic and optical errors, thus enhancing fusion in patients [26-29]. As a result, providing a clearer retinal image increases the range of high-quality foveation gaze, benefits that cannot be solely evaluated by evaluating the visual acuity in the primary position [15]. Overall, CIs are associated with an increased field of vision and improved binocular vision [30].
There is no consensus regarding the effect of CLs on visual acuity. Studies have shown that CLs can improve visual acuity [9, 13-17, 26, 31, 32], have no effect on it [8, 33], or, in some cases, reduce visual acuity [33]. However, visual acuity is not the only functional factor in vision, and sometimes patients prefer to use CLs even without improvement in visual acuity, reporting an increase in the quality of their vision [8, 13].
It is important to remember that soft CLs cannot fully correct high astigmatisms when wearing CLs. Hard CLs are necessary to correct refractive errors. With CLs, the retinal image becomes significantly less minified, which occurs when wearing glasses to correct myopia. Hence, an increase in the retinal image's size is the overall effect of wearing CLs in myopes [34]. This suggests that the improvement in visual acuity observed in IN patients may be related to the use of CLs and retinal image magnification in myopic individuals [14, 17, 30, 31].
On the other hand, for individuals with high hyperopia and aphakia, contact lens (CL) correction leads to a smaller retinal image compared to glasses correction, which can result in a slight decrease in visual acuity with CIs [34]. As a result, in these people, improvement in visual acuity in IN due to image magnification is not expected.
Mechanical and sensory effect of CL on nystagmus intensity
The nystagmus waveform represents the eye position over time and is obtained by recording eye movements, which are described by the amplitude, frequency, and waveform [35]. The intensity of nystagmus is calculated by multiplying the amplitude of nystagmus by the frequency, indicating the average speed of eye movements [36]. Variations in nystagmus intensity are observed with different viewing angles; the angle exhibiting the least intensity is referred to as the "null position." When the null position is not in the primary position, individuals may assume an atypical head position in order to position their eyes in that manner.
The effect of CLs on the severity of nystagmus can be discussed from several perspectives. The first aspect is the effect on the amplitude of nystagmus movements, which reduces the amplitude of nystagmus movements [9-11, 13, 14, 26, 32, 37, 38]. Except in a study which were ineffective [33] or in one case increased the amplitude [13]. Another aspect is the effect on the frequency of nystagmus, which in some CLs reduces the frequency of nystagmus [9, 14, 17, 26, 32] and it has no effect in others [13, 33].
In general, the intensity of nystagmus also decreases in people whose amplitude and frequency have decreased. This reduction is notable even when the amplitude is reduced but the frequency remains unchanged, suggesting an increase in the foveation period due to reduced image movements on the retina [14]. Therefore, in addition to improving visual acuity in the initial position, CLs expand the high-quality Foveation gaze angle. Consequently, CLs provide a wider field of high foveation quality and improved foveation at each gaze angle [38]. However, considering that in some cases the range of nystagmus may increase, CLs might not be suitable for everyone, and other factors should be considered.
Anesthetic drops can be used to evaluate the effect of CLs on nerve endings. The CL impacts eye movements and dampens nystagmus movements across various viewing angles and waveforms by stimulating the afferent pathways of the ophthalmic division of the trigeminal cranial nerve and maintaining constant contact with the cornea and eyelid [15, 39]. By using topical anesthetic drops, we eliminate the mechanical effect of the CL on the sensory nerve endings, and if the sole functional factor is the effect on the nerve endings, this effect should disappear.
Despite the use of a local anesthetic drop on the cornea, along with a contact lens, the intensity of nystagmus lessens and does not fully return to its initial level without the contact lens. Additionally, the positive effects of the contact lens do not fade away [14, 40]. However, the findings of the Dell'Osso trial contradict this, as they showed that the use of local anesthetic drops eliminated this impact and restored it to its original level (without CLs) [39].
The utilization of Plano soft CLs eliminates the potential for enhancing visual acuity through CLs. Instead, the impact on nerve terminals is achieved, leading us to deduce that the reduction in nystagmus movements with CLs is a result of the mechanical influence exerted by the lenses on the nerve terminals [9, 11, 37]. During this assessment, the application of an anesthetic drop on the Plano CL amplifies the intensity of nystagmus but does not revert to its initial level [9, 37]. This issue can be due to the mechanism of the effect of the anesthetic drop or the placebo factor or motivation on the intensity of nystagmus.
It should also be considered that soft CLs were used in the study, in which the damping effect of CLs was lost with drops, which could be caused by the type of soft lens material in creating a mechanical effect on nerve terminals, which is not mentioned in the article [39]. Nonetheless, we observed positive results with the soft lens, which did not return to the initial level [11, 37]. Meanwhile, studies on hard lenses and the effect of anesthetic drops have shown that the positive effects of the CL are not merely negated by the drops [10]. The evidence shows that the therapeutic effect of CLs on IN is not only due to their ability to correct refractive errors or only the effect on the trigeminal nerve, but it can also be due to their interference with neural mechanisms affecting the characteristics of nystagmus at higher levels.
Attenuating IN does not necessarily improve acuity. The most significant effects of treatment are prolonging the foveation period and reducing positional changes, which enhance performance and aesthetics.
It seems that the presence of CLs on the eye reduces the severity of nystagmus and leads to a change in the waveform, but when the eye is numb, the waveform returns to its original state. This suggests that the primary mechanism involved may not be refractive error correction but rather sensory feedback from the CLs on the cornea or eyelids, which may modulate nystagmus.
Contact lens and convergence relationship
In people with IN, whose null potion is present in a certain angle of view, using base-out or base-in (BI) prisms to align their eyes to that angle can reduce nystagmus movements [24, 27]. Also, if nystagmus intensity decreases with convergence during close vision, using a base-out prism to maintain eyes in a convergence mode while looking together [41, 42] can help lessen nystagmus intensity and also improve visual acuity [43].
It is worth mentioning that reducing the range of nystagmus movements is not an accurate measure for improving visual acuity but is more beneficial aesthetically. Basically, prolonging the period of foveation and reducing positional variations are crucial treatment effects for enhancing performance [43]. Convergence in congenital nystagmus by expanding and improving the range of high-acuity gaze angles [44] enables the patient to see better and more broadly. This means that foveation improves across different gazes, and its range becomes wider.
When using CLs, myopic individuals experience greater convergence while focusing up close compared to when they use glasses. Basically, negative glasses work like BI relieving prism and make the eye less convergent.
As a result, the use of CLs in myopic people increases convergence [45]. It is important to consider whether individuals are myopic or hyperopic when assessing the outcomes of studies on the effectiveness of CLs. If you are myopic and use a contact lens, in addition to the contact effect and correction of the refractive error by the CL and increasing the foveation period, especially in people with an eccentric null point, the issue of convergence and reduction of eye movements should also be considered. As expected, in studies that specifically evaluated myopic people, CLs could reduce nystagmus [8, 9, 10, 14, 32] and improve visual acuity [9, 14, 17, 30, 31], except in a case, where myopia was accompanied by isotropia [13], which leads to increased adaptive vergence, the esotropia range widens, binocular vision becomes more unstable, and recovery is not achieved.
Conversely, positive glasses lenses act as base-out prisms for hyperopic individuals when viewing objects up close, thereby inducing more convergence than when using CLs. Therefore, a person who wears hyperopia has less convergence and compatibility with CLs compared to wearing glasses.
So, in hyperopia, a hyperopic person wearing CLs exhibits less convergence and adaptability compared to wearing glasses. In hyperopia without any associated deviation, CLs may not be beneficial and could potentially worsen the situation. If hyperopia is coupled with exotropia, a reduction in nystagmus movements is expected, especially in severe cases. As a result, it is expected that in the studies on hyperopes, CLs do not have a significant effect on reducing eye movements [13, 15, 16] and only in one study, the severity of nystagmus was reduced [26]. As a result, the role of convergence is not significant in these people, and any improvement in visual acuity is only due to enhanced vision and the mechanical effect of using hard lenses [15, 26, 31].
Relationship between CL and reading performance
Clinically, the reading rate is evaluated by determining the correct word reading in one minute, which depends on the types of optical correction and reading material [46]. The relationship between reading performance and the degree of sensory and motor impairment in neonatal nystagmus is not clear. The subject can be examined from two sensory and movement points of view. From the ocular motor perspective, nystagmus directly affects the accuracy and speed of saccadic and fixation movements during reading [47] and can reduce reading speed [48]. From a sensory perspective, afferent defects in congenital nystagmus can directly affect reading performance.
Studies have investigated whether nystagmus affects people’s ability to read. Some believe that nystagmus slows down reading compared to normal people. By examining the reading performance of 71 people with IN and 20 normal people, Barot et al. found that those with nystagmus related to albinism read 18.8% slower and those with idiopathic nystagmus read 14.7% slower than those without nystagmus [49]. However, they noted that near-normal reading speed can be achieved with font sizes up to 0.6 log MAR larger than near VA [50]. While it has been reported in some articles that people with IN study at a normal speed [51] or close to normal [47-49, 51].
The question now is whether CLs improve reading performance in nystagmus patients. A study on congenital nystagmus found that the use of soft CLs increases reading speed [9, 13, 17], with some cases showing a doubling of reading speed [33]. However, in Jayaramachandran et al.’s study, while reading speed with CLs did not improve, in some cases using soft CLs resulted in a decrease in reading speed [17]. This is while in Jayaramachandran's study, in addition to the fact that reading speed with CLs did not improve, in some cases of using soft CLs, reading speed decreased [33], which can be seen as related to the lack of separation of patients with myopia and hyperopia and the effects Be careful about these factors. It should be noted that although reading performance is a very important and critical issue, especially in school age, but due to the small number of studies in this field, it is not possible to obtain a definitive result on the effect of CLs on the functional ability of reading.
The relationship between CLs and contrast sensitivity
Contrast sensitivity is considered one of the most reliable measures for evaluating spatial processing and visual functions. It develops from birth to a fully mature state between the ages of 8 to 19. The quality of contrast sensitivity is influenced by two factors, the retina and the cerebral cortex. Therefore, it relies on the maturation of the visual system. Contrast sensitivity has also been shown to decrease with age. Other factors affecting contrast sensitivity performance (CSF) include target spatial parameters such as size and color, orientation, luminance, presentation time, and eye movements.
Visual acuity test has the ability to evaluate spatial resolution related to visual performance. Although visual acuity is often used as the main measure of visual functions, it should be kept in mind that it is not able to fully evaluate visual functional abilities. Some vision disorders exhibit defects in contrast sensitivity despite reporting normal visual acuity.
Contrast sensitivity is mostly used as a tool to test aspects of visual function. IN affects spatial-temporal visual functions due to spontaneous oscillatory eye movements, resulting in decreased contrast sensitivity.
By assessing contrast sensitivity with sinusoidal gratings in individuals with nystagmus who were wearing hard CLs, an enhancement in contrast sensitivity was observed. It is suggested that evaluating these individuals based on contrast sensitivity is preferable to utilizing the Snellen chart to determine visual acuity [9], which has been confirmed in some other studies [13, 14, 26]. It should be noted that all the articles that have observed an improvement in contrast sensitivity agree on its effect at low frequencies. However, the results differ at high sensitivities [26].
The relationship between CIs and QoL
A very important point in the success of using CLs in all people is the motivation of the patient. The mental state of the person with nystagmus affects the intensity of nystagmus [40, 52, 53]. If a person does not want to use CLs, it can become a source of stress for the person and lead to an increase in the intensity of nystagmus. In contrast, when the patient is highly motivated when she/he considers it effective in the form of a placebo and gives him/her peace of mind, it affects the mental state of the person and can affect the results and reduce the intensity of nystagmus. Only one study assessed the performance of soft lenses using the QoL VFQ-25 questionnaire [13], and initially, patients who did not have sufficient motivation or desire to wear lenses were excluded from the study. His exclusion could introduce bias, especially in responses to lifestyle questionnaires, potentially skewing results in favor of CL practicality. However, it is worth noting that most relevant studies have subjectively reported improved patient satisfaction with their eye condition after using CLs, prompting questions about other aspects of eye examination beyond the effectiveness of CLs.
Conclusion
In most studies, no side effect of using CLs has been reported, except in rare cases where oscillopsia increases transiently after CL removal. This is despite the fact that most of the patients have obtained similar or better results in visual performance with the use of glasses. Therefore, if CLs are not considered superior to glasses, comparable performance can still be expected, making them a viable first-line treatment option for patients with indications for their prescription. It is crucial to prioritize safety, with glasses being the primary choice in this regard. Therefore, thorough patient assessment before fitting is necessary to weigh the advantages and disadvantages of CLs on an individual basis. Nevertheless, considering that most patients have reported positive effects on their QoL with CLs, they can be recommended as part of the treatment regimen.
At first, one might underestimate the significance of improving visual acuity by one or more lines or the minor enhancement in reading performance concerning patients’ QoL. However, let’s consider a young adult who legally needs a one-line improvement in his visual acuity to receive a certificate or a child who at school age cannot show all his/her talents due to low reading performance, leading to pressure and limitations in school activities. How might these situations affect their lives in the long term? Even seemingly small improvements can have a profound impact, which is why patients often report satisfaction with even minor enhancements observed in studies. Therefore, there is little room for complacency in this regard. Furthermore, the last point is that we should keep in mind that the effectiveness of CLs, particularly when initiated at a young age, can significantly influence vision development and potentially reduce the prevalence of amblyopia (lazy eye) in affected individuals, thereby positively impacting their lives.
As previous study demonstrated, CLs are much more useful for improving visual function than previously thought and are a potentially important treatment option for IN patients.
Ethical Considerations
Compliance with ethical guidelines
This research did not involve the use of human volunteers or animals, ensuring compliance with ethical guidelines.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the Optometry Department of Iran University of Medical Sciences for their cooperation and help in designing the study.
References
Infantile nystagmus (IN) is an involuntary rhythmic oscillation of the eyes that usually occurs in the first 3 to 6 months of life [1] and its prevalence in the general population is reported to be 0.14% [2]. This disorder is classically divided into afferent nystagmus (sensory defect) and efferent nystagmus (idiopathic). Afferent nystagmus mainly occurs in the first few months of life due to insufficient image formation in the fovea, which is caused by anatomical changes or organic defects in the eye [3]. Among the most common causes of afferent nystagmus are eye diseases, such as congenital cataracts, albinism, and corneal opacity, as well as developmental abnormalities of the optic disc and retina such as Leber’s congenital amaurosis, achromatopsia or congenital stationary night blindness [4]. Efferent or idiopathic nystagmus, on the other hand, is a motor functional disorder that occurs without any ocular or systemic pathology [5].
Nystagmus, with a lifelong negative effect on the quality of life (QoL) of patients, leads to an increase in their dependence on others and a decrease in self-confidence and social relationships [6]. Effective management and treatment are crucial for enhancing visual function and social engagement. The objectives of nystagmus treatment include optimizing vision by increasing foveation periods, reducing nystagmus severity, correcting abnormal head positions, and addressing strabismus [7]. Non-surgical management strategies for nystagmus include medications, biofeedback techniques, acupuncture, prismatic glasses, and contact lenses (CLs). This article focuses on assessing the impact of CLs on this disorder.
Research has evaluated the effectiveness and performance of both soft and rigid gas permeable (RGP) CLs on nystagmus, noting that each type may improve visual performance due to its distinct characteristics.
The first goal of this study was to systematically review the literature for evidence that shows the effect of using CLs on the visual performance of a person with congenital nystagmus and then to find the differences between soft and hard CLs. Our secondary goal was to create a framework to better understand the advantages and disadvantages of using CLs in people with congenital nystagmus for the clinical management of patients with IN. As a result, this review article examined the evidence available in the medical literature in response to these questions.
Methods
At first, we looked for studies on the effect of hard and soft CLs on congenital nystagmus patients by reviewing the literature.
The proposed systematic review will answer the following question:
Do IN patients who wear hard CLs have better vision than those who wear soft CLs?
Search strategy
This systematic review was conducted according to the guidelines set forth in the statement of preferred reporting items for systematic reviews and meta-analyses (PRISMA). We searched PubMed, Scopus, Google Scholar, and the Cochrane Central Register of Controlled Trials for relevant studies from inception to May 9, 2022. We excluded review articles and imposed no restrictions on publication time or language. The search utilized medical subject headings (MeSH) and keyword combinations of nystagmus, contact lens, congenital nystagmus, IN, rigid gas permeable lenses, and soft CLs. Boolean operators “AND” and “OR” were used to refine the search.
Eligibility criteria
Inclusion criteria
Inclusion criteria were formulated using the participants, intervention, comparison, outcomes, and study designs (PICOS) strategy. Studies were selected based on the following criteria: 1) Type of study: All types of studies available based on the PRISMA criteria in the field of IN, 2) Participants: All individuals with neonatal nystagmus, 3) Intervention: The use of soft or hard CLs in patients with congenital nystagmus, 4) Outcomes: Improvement of visual functions, including visual acuity, contrast sensitivity, and reading performance, as well as improvements in the intensity, range, and frequency of eye movements. The inclusion criteria are given in Table 1.

Exclusion criteria
Given the scarcity of studies in this area, the exclusion criteria were minimal, excluding only animal studies and studies involving patients who underwent various types of surgery prior to the intervention.
Quality assessment
The quality of the studies was evaluated using the JBI critical appraisal tool checklist for case series and case reports (Table 2).

Factors evaluated for potential bias included clarity and impartiality of inclusion criteria, the objectivity of exposure assessment (the condition measured), the employment of valid methods, comprehensive reporting of demographic profiles, completeness of case information, and the appropriateness of the statistical analyses used.
Results
A total of 38 articles were identified in PubMed using MeSH keywords, and 8 articles were found in Scopus review. From the Cochrane Central Register of Controlled Trials (CENTRAL), 9 articles were identified, with 2 overlapping with those found in Scopus. In Google Scholar, after utilizing advanced search criteria on titles and abstracts, 23 articles were identified, and an additional 6 articles were found. A list of other related articles was compiled through reference checking.
Initially, out of 102 articles found, after removing duplicate articles, 76 articles remained, and then in the screening, 39 articles were removed from the remaining 76 articles due to being unrelated and 8 articles due to the lack of access to the abstract or full text. A total of 29 articles were included in this systematic review. The process of identifying studies is shown in Figure 1. Table 3 shows the characteristics and results of the studies.



Data extraction
After completing the database search and removing duplicates by title check, titles, and abstracts were screened to identify potentially eligible articles. Full texts of these articles were then reviewed to ensure they met the inclusion and exclusion criteria. For the included studies, data were extracted on study characteristics (authors, year, study design, follow-up time, interventions, and controls), patient characteristics (age, changes in visual acuity, changes in intensity, frequency of nystagmus, and contrast sensitivity).
Discussion
The application of CLs in patients with congenital nystagmus has been documented in scientific literature since 1962 [18], yet research in this area remains sparse, and the few existing studies have yielded conflicting results.
There is only one RCT study in this field. Aside from the one RCT in this field, the vast majority of research consists of case reports with small sample sizes. Additionally, these studies generally involve short-term follow-ups and overall, do not possess strong methodologies. As a result, we decided to have a complete review of the articles in this field. Initially, it is essential to determine which mechanisms most likely contribute to the effects of CLs on IN.
Refractive errors and nystagmus
Considering the higher prevalence of refractive errors in people with IN compared to the general population [19] and the wide range of refractive errors found in these patients, correcting refractive errors in these people is important. Even minimal corrections can improve visual perception. The incidence of corneal astigmatism is notably high in individuals with IN [20-22], with 57% experiencing astigmatism greater than 2.00 diopters [21]. Studies suggest that astigmatism increases with age [23] and is likely caused by continuous interactions between the cornea and eyelid during eye movements, which predominantly occur along the horizontal axis, although vertical or torsional movements may also be present [22, 24]. There is no consensus about the prevalence of myopia [21] and hyperopia [25] in different studies.
The initial step in managing IN involves correcting refractive errors, typically through glasses or CLs. For high refractive errors, glasses pose challenges due to constant eye movement, preventing consistent focus through the optical center of the glasses [26]. As a result, when patients look through areas other than the optical center, they experience prismatic effects, spherical, and chromatic aberrations, which interfere with the foveation period and impair sensory system function. In contrast, CLs move with the eyes, ensuring optimal visual acuity through the optical center at all times. They minimize adverse effects associated with glasses, such as magnification and minification, particularly in cases of high refractive errors, and reduce prismatic and optical errors, thus enhancing fusion in patients [26-29]. As a result, providing a clearer retinal image increases the range of high-quality foveation gaze, benefits that cannot be solely evaluated by evaluating the visual acuity in the primary position [15]. Overall, CIs are associated with an increased field of vision and improved binocular vision [30].
There is no consensus regarding the effect of CLs on visual acuity. Studies have shown that CLs can improve visual acuity [9, 13-17, 26, 31, 32], have no effect on it [8, 33], or, in some cases, reduce visual acuity [33]. However, visual acuity is not the only functional factor in vision, and sometimes patients prefer to use CLs even without improvement in visual acuity, reporting an increase in the quality of their vision [8, 13].
It is important to remember that soft CLs cannot fully correct high astigmatisms when wearing CLs. Hard CLs are necessary to correct refractive errors. With CLs, the retinal image becomes significantly less minified, which occurs when wearing glasses to correct myopia. Hence, an increase in the retinal image's size is the overall effect of wearing CLs in myopes [34]. This suggests that the improvement in visual acuity observed in IN patients may be related to the use of CLs and retinal image magnification in myopic individuals [14, 17, 30, 31].
On the other hand, for individuals with high hyperopia and aphakia, contact lens (CL) correction leads to a smaller retinal image compared to glasses correction, which can result in a slight decrease in visual acuity with CIs [34]. As a result, in these people, improvement in visual acuity in IN due to image magnification is not expected.
Mechanical and sensory effect of CL on nystagmus intensity
The nystagmus waveform represents the eye position over time and is obtained by recording eye movements, which are described by the amplitude, frequency, and waveform [35]. The intensity of nystagmus is calculated by multiplying the amplitude of nystagmus by the frequency, indicating the average speed of eye movements [36]. Variations in nystagmus intensity are observed with different viewing angles; the angle exhibiting the least intensity is referred to as the "null position." When the null position is not in the primary position, individuals may assume an atypical head position in order to position their eyes in that manner.
The effect of CLs on the severity of nystagmus can be discussed from several perspectives. The first aspect is the effect on the amplitude of nystagmus movements, which reduces the amplitude of nystagmus movements [9-11, 13, 14, 26, 32, 37, 38]. Except in a study which were ineffective [33] or in one case increased the amplitude [13]. Another aspect is the effect on the frequency of nystagmus, which in some CLs reduces the frequency of nystagmus [9, 14, 17, 26, 32] and it has no effect in others [13, 33].
In general, the intensity of nystagmus also decreases in people whose amplitude and frequency have decreased. This reduction is notable even when the amplitude is reduced but the frequency remains unchanged, suggesting an increase in the foveation period due to reduced image movements on the retina [14]. Therefore, in addition to improving visual acuity in the initial position, CLs expand the high-quality Foveation gaze angle. Consequently, CLs provide a wider field of high foveation quality and improved foveation at each gaze angle [38]. However, considering that in some cases the range of nystagmus may increase, CLs might not be suitable for everyone, and other factors should be considered.
Anesthetic drops can be used to evaluate the effect of CLs on nerve endings. The CL impacts eye movements and dampens nystagmus movements across various viewing angles and waveforms by stimulating the afferent pathways of the ophthalmic division of the trigeminal cranial nerve and maintaining constant contact with the cornea and eyelid [15, 39]. By using topical anesthetic drops, we eliminate the mechanical effect of the CL on the sensory nerve endings, and if the sole functional factor is the effect on the nerve endings, this effect should disappear.
Despite the use of a local anesthetic drop on the cornea, along with a contact lens, the intensity of nystagmus lessens and does not fully return to its initial level without the contact lens. Additionally, the positive effects of the contact lens do not fade away [14, 40]. However, the findings of the Dell'Osso trial contradict this, as they showed that the use of local anesthetic drops eliminated this impact and restored it to its original level (without CLs) [39].
The utilization of Plano soft CLs eliminates the potential for enhancing visual acuity through CLs. Instead, the impact on nerve terminals is achieved, leading us to deduce that the reduction in nystagmus movements with CLs is a result of the mechanical influence exerted by the lenses on the nerve terminals [9, 11, 37]. During this assessment, the application of an anesthetic drop on the Plano CL amplifies the intensity of nystagmus but does not revert to its initial level [9, 37]. This issue can be due to the mechanism of the effect of the anesthetic drop or the placebo factor or motivation on the intensity of nystagmus.
It should also be considered that soft CLs were used in the study, in which the damping effect of CLs was lost with drops, which could be caused by the type of soft lens material in creating a mechanical effect on nerve terminals, which is not mentioned in the article [39]. Nonetheless, we observed positive results with the soft lens, which did not return to the initial level [11, 37]. Meanwhile, studies on hard lenses and the effect of anesthetic drops have shown that the positive effects of the CL are not merely negated by the drops [10]. The evidence shows that the therapeutic effect of CLs on IN is not only due to their ability to correct refractive errors or only the effect on the trigeminal nerve, but it can also be due to their interference with neural mechanisms affecting the characteristics of nystagmus at higher levels.
Attenuating IN does not necessarily improve acuity. The most significant effects of treatment are prolonging the foveation period and reducing positional changes, which enhance performance and aesthetics.
It seems that the presence of CLs on the eye reduces the severity of nystagmus and leads to a change in the waveform, but when the eye is numb, the waveform returns to its original state. This suggests that the primary mechanism involved may not be refractive error correction but rather sensory feedback from the CLs on the cornea or eyelids, which may modulate nystagmus.
Contact lens and convergence relationship
In people with IN, whose null potion is present in a certain angle of view, using base-out or base-in (BI) prisms to align their eyes to that angle can reduce nystagmus movements [24, 27]. Also, if nystagmus intensity decreases with convergence during close vision, using a base-out prism to maintain eyes in a convergence mode while looking together [41, 42] can help lessen nystagmus intensity and also improve visual acuity [43].
It is worth mentioning that reducing the range of nystagmus movements is not an accurate measure for improving visual acuity but is more beneficial aesthetically. Basically, prolonging the period of foveation and reducing positional variations are crucial treatment effects for enhancing performance [43]. Convergence in congenital nystagmus by expanding and improving the range of high-acuity gaze angles [44] enables the patient to see better and more broadly. This means that foveation improves across different gazes, and its range becomes wider.
When using CLs, myopic individuals experience greater convergence while focusing up close compared to when they use glasses. Basically, negative glasses work like BI relieving prism and make the eye less convergent.
As a result, the use of CLs in myopic people increases convergence [45]. It is important to consider whether individuals are myopic or hyperopic when assessing the outcomes of studies on the effectiveness of CLs. If you are myopic and use a contact lens, in addition to the contact effect and correction of the refractive error by the CL and increasing the foveation period, especially in people with an eccentric null point, the issue of convergence and reduction of eye movements should also be considered. As expected, in studies that specifically evaluated myopic people, CLs could reduce nystagmus [8, 9, 10, 14, 32] and improve visual acuity [9, 14, 17, 30, 31], except in a case, where myopia was accompanied by isotropia [13], which leads to increased adaptive vergence, the esotropia range widens, binocular vision becomes more unstable, and recovery is not achieved.
Conversely, positive glasses lenses act as base-out prisms for hyperopic individuals when viewing objects up close, thereby inducing more convergence than when using CLs. Therefore, a person who wears hyperopia has less convergence and compatibility with CLs compared to wearing glasses.
So, in hyperopia, a hyperopic person wearing CLs exhibits less convergence and adaptability compared to wearing glasses. In hyperopia without any associated deviation, CLs may not be beneficial and could potentially worsen the situation. If hyperopia is coupled with exotropia, a reduction in nystagmus movements is expected, especially in severe cases. As a result, it is expected that in the studies on hyperopes, CLs do not have a significant effect on reducing eye movements [13, 15, 16] and only in one study, the severity of nystagmus was reduced [26]. As a result, the role of convergence is not significant in these people, and any improvement in visual acuity is only due to enhanced vision and the mechanical effect of using hard lenses [15, 26, 31].
Relationship between CL and reading performance
Clinically, the reading rate is evaluated by determining the correct word reading in one minute, which depends on the types of optical correction and reading material [46]. The relationship between reading performance and the degree of sensory and motor impairment in neonatal nystagmus is not clear. The subject can be examined from two sensory and movement points of view. From the ocular motor perspective, nystagmus directly affects the accuracy and speed of saccadic and fixation movements during reading [47] and can reduce reading speed [48]. From a sensory perspective, afferent defects in congenital nystagmus can directly affect reading performance.
Studies have investigated whether nystagmus affects people’s ability to read. Some believe that nystagmus slows down reading compared to normal people. By examining the reading performance of 71 people with IN and 20 normal people, Barot et al. found that those with nystagmus related to albinism read 18.8% slower and those with idiopathic nystagmus read 14.7% slower than those without nystagmus [49]. However, they noted that near-normal reading speed can be achieved with font sizes up to 0.6 log MAR larger than near VA [50]. While it has been reported in some articles that people with IN study at a normal speed [51] or close to normal [47-49, 51].
The question now is whether CLs improve reading performance in nystagmus patients. A study on congenital nystagmus found that the use of soft CLs increases reading speed [9, 13, 17], with some cases showing a doubling of reading speed [33]. However, in Jayaramachandran et al.’s study, while reading speed with CLs did not improve, in some cases using soft CLs resulted in a decrease in reading speed [17]. This is while in Jayaramachandran's study, in addition to the fact that reading speed with CLs did not improve, in some cases of using soft CLs, reading speed decreased [33], which can be seen as related to the lack of separation of patients with myopia and hyperopia and the effects Be careful about these factors. It should be noted that although reading performance is a very important and critical issue, especially in school age, but due to the small number of studies in this field, it is not possible to obtain a definitive result on the effect of CLs on the functional ability of reading.
The relationship between CLs and contrast sensitivity
Contrast sensitivity is considered one of the most reliable measures for evaluating spatial processing and visual functions. It develops from birth to a fully mature state between the ages of 8 to 19. The quality of contrast sensitivity is influenced by two factors, the retina and the cerebral cortex. Therefore, it relies on the maturation of the visual system. Contrast sensitivity has also been shown to decrease with age. Other factors affecting contrast sensitivity performance (CSF) include target spatial parameters such as size and color, orientation, luminance, presentation time, and eye movements.
Visual acuity test has the ability to evaluate spatial resolution related to visual performance. Although visual acuity is often used as the main measure of visual functions, it should be kept in mind that it is not able to fully evaluate visual functional abilities. Some vision disorders exhibit defects in contrast sensitivity despite reporting normal visual acuity.
Contrast sensitivity is mostly used as a tool to test aspects of visual function. IN affects spatial-temporal visual functions due to spontaneous oscillatory eye movements, resulting in decreased contrast sensitivity.
By assessing contrast sensitivity with sinusoidal gratings in individuals with nystagmus who were wearing hard CLs, an enhancement in contrast sensitivity was observed. It is suggested that evaluating these individuals based on contrast sensitivity is preferable to utilizing the Snellen chart to determine visual acuity [9], which has been confirmed in some other studies [13, 14, 26]. It should be noted that all the articles that have observed an improvement in contrast sensitivity agree on its effect at low frequencies. However, the results differ at high sensitivities [26].
The relationship between CIs and QoL
A very important point in the success of using CLs in all people is the motivation of the patient. The mental state of the person with nystagmus affects the intensity of nystagmus [40, 52, 53]. If a person does not want to use CLs, it can become a source of stress for the person and lead to an increase in the intensity of nystagmus. In contrast, when the patient is highly motivated when she/he considers it effective in the form of a placebo and gives him/her peace of mind, it affects the mental state of the person and can affect the results and reduce the intensity of nystagmus. Only one study assessed the performance of soft lenses using the QoL VFQ-25 questionnaire [13], and initially, patients who did not have sufficient motivation or desire to wear lenses were excluded from the study. His exclusion could introduce bias, especially in responses to lifestyle questionnaires, potentially skewing results in favor of CL practicality. However, it is worth noting that most relevant studies have subjectively reported improved patient satisfaction with their eye condition after using CLs, prompting questions about other aspects of eye examination beyond the effectiveness of CLs.
Conclusion
In most studies, no side effect of using CLs has been reported, except in rare cases where oscillopsia increases transiently after CL removal. This is despite the fact that most of the patients have obtained similar or better results in visual performance with the use of glasses. Therefore, if CLs are not considered superior to glasses, comparable performance can still be expected, making them a viable first-line treatment option for patients with indications for their prescription. It is crucial to prioritize safety, with glasses being the primary choice in this regard. Therefore, thorough patient assessment before fitting is necessary to weigh the advantages and disadvantages of CLs on an individual basis. Nevertheless, considering that most patients have reported positive effects on their QoL with CLs, they can be recommended as part of the treatment regimen.
At first, one might underestimate the significance of improving visual acuity by one or more lines or the minor enhancement in reading performance concerning patients’ QoL. However, let’s consider a young adult who legally needs a one-line improvement in his visual acuity to receive a certificate or a child who at school age cannot show all his/her talents due to low reading performance, leading to pressure and limitations in school activities. How might these situations affect their lives in the long term? Even seemingly small improvements can have a profound impact, which is why patients often report satisfaction with even minor enhancements observed in studies. Therefore, there is little room for complacency in this regard. Furthermore, the last point is that we should keep in mind that the effectiveness of CLs, particularly when initiated at a young age, can significantly influence vision development and potentially reduce the prevalence of amblyopia (lazy eye) in affected individuals, thereby positively impacting their lives.
As previous study demonstrated, CLs are much more useful for improving visual function than previously thought and are a potentially important treatment option for IN patients.
Ethical Considerations
Compliance with ethical guidelines
This research did not involve the use of human volunteers or animals, ensuring compliance with ethical guidelines.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the Optometry Department of Iran University of Medical Sciences for their cooperation and help in designing the study.
References
- Hertle RW. Examination and refractive management of patients with nystagmus. Surv Ophthalmol. 2000; 45(3):215-22. [DOI:10.1016/S0039-6257(00)00153-3] [PMID]
- Sarvananthan N, Surendran M, Roberts EO, Jain S, Thomas S, Shah N, et al. The prevalence of nystagmus: The Leicestershire nystagmus survey. Invest Ophthalmol Vis Sci. 2009; 50(11):5201-6. [DOI:10.1167/iovs.09-3486] [PMID]
- Weiss AH, Biersdorf WR. Visual sensory disorders in congenital nystagmus. Ophthalmology. 1989; 96(4):517-23. [DOI:10.1016/S0161-6420(89)32864-8] [PMID]
- Pearce WG. Congenital nystagmus--genetic and environmental causes. Can J Ophthalmol. 1978; 13(1):1-9. [PMID]
- Mravičić I, Lukačević S, Bohač M, Pauk-Gulić M, Glavota V. Nystagmus. Eye Motility. London: IntechOen; 2019. [Link]
- Decarlo DK, McGwin G Jr, Bixler ML, Wallander J, Owsley C. Impact of pediatric vision impairment on daily life: Results of focus groups. Optom Vis Sci. 2012; 89(9):1409-16. [DOI:10.1097/OPX.0b013e318264f1dc] [PMID]
- Papageorgiou E, Lazari K, Gottlob I. The challenges faced by clinicians diagnosing and treating infantile nystagmus Part II: Treatment. Expert Review of Ophthalmology. 2021; 16(6):449-65. [DOI; 10.1080/17469899.2021.1970533]
- Enoch JM, Windsor CE. Remission of nystagmus following fitting contact lenses to an infant with aniridia. Am J Ophthalmol. 1968; 66(2):333-5. [DOI:10.1016/0002-9394(68)92084-9] [PMID]
- Abadi RV. Mechanisms underlying nystagmus. J R Soc Med. 2002; 95(5):231-4. [DOI:10.1177/014107680209500504] [PMID]
- Abel LA, Williams IM, Levi L. Intermittent oscillopsia in a case of congenital nystagmus. Dependence upon waveform. Invest Ophthalmol Vis Sci. 1991; 32(12):3104-8. [PMID]
- Safran AB, Gambazzi Y. Congenital nystagmus: Rebound phenomenon following removal of contact lenses. Br J Ophthalmol. 1992; 76(8):497-8. [DOI:10.1136/bjo.76.8.497] [PMID]
- Leung V, Wick B, Bedell HE. Multifaceted treatment of congenital nystagmus: a report of 6 cases. Optom Vis Sci. 1996; 73(2):114-24. [DOI: 10.1097/00006324-199602000-00007] [PMID]
- Biousse V, Tusa RJ, Russell B, Azran MS, Das V, Schubert MS, et al. The use of contact lenses to treat visually symptomatic congenital nystagmus. J Neurol Neurosurg Psychiatry. 2004; 75(2):314-6. [DOI:10.1136/jnnp.2003.010678] [PMID]
- Rutner D, Ciuffreda K. Soft contact lenses to improve motor and sensory function in congenital nystagmus. J Behav Optom. 2005; 16(1):17-20. [Link]
- Taibbi G, Wang ZI, Dell'Osso LF. Infantile nystagmus syndrome: Broadening the high-foveation-quality field with contact lenses. Clin Ophthalmol. 2008; 2(3):585-9. [DOI:10.2147/OPTH.S2744] [PMID]
- Stevenson G, Gardner L. Progressive cone dystrophy, nystagmus and contact lenses. Cont Lens Anterior Eye. 2010; 33(5):228-30. [DOI:10.1016/j.clae.2010.02.006] [PMID]
- Shahimin M, Mohammed Z, Saliman N, Mohamad-Fadzil N, Razali N, Mutalib H, et al. The use of an infrared eye tracker in evaluating the reading performance in a congenital nystagmus patient fitted with soft contact lens: A case report. In: Horsley M, Eliot M, Knight B, Reilly R, editors. Current trends in eye tracking research. Cham: Springer; 2014. [DOI:10.1007/978-3-319-02868-2_8]
- Hale JR. Contact-lens application in four cases of congenital nystagmus. Optom Wkly. 1962; 53:1865-8. [PMID]
- Gottlob I. Is impaired emmetropization related to foveal hypoplasia or is it specific to albinism? Invest Ophthalmol Vis Sci. 2013; 54(4):2940. [DOI:10.1167/iovs.13-12097] [PMID]
- Reinecke RD. Costenbader lecture. Idiopathic infantile nystagmus: Diagnosis and treatment. J AAPOS. 1997; 1(2):67-82. [DOI:10.1016/S1091-8531(97)90002-1] [PMID]
- Sampath V, Bedell HE. Distribution of refractive errors in albinos and persons with idiopathic congenital nystagmus. Optom Vis Sci. 2002; 79(5):292-9. [DOI:10.1097/00006324-200205000-00008] [PMID]
- Wang J, Wyatt LM, Felius J, Stager DR Jr, Stager DR Sr, Birch EE, et al. Onset and progression of with-the-rule astigmatism in children with infantile nystagmus syndrome. Invest Ophthalmol Vis Sci. 2010; 51(1):594-601. [DOI:10.1167/iovs.09-3599] [PMID]
- Fresina M, Benedetti C, Marinelli F, Versura P, Campos EC. Astigmatism in patients with idiopathic congenital nystagmus. Graefes Arch Clin Exp Ophthalmol. 2013; 251(6):1635-9. [DOI:10.1007/s00417-013-2290-y] [PMID]
- Dickinson CM. The elucidation and use of the effect of near fixation in congenital nystagmus. Ophthalmic Physiol Opt. 1986; 6(3):303-11. [DOI:10.1111/j.1475-1313.1986.tb00720.x] [PMID]
- Healey N, McClelland JF, Saunders KJ, Jackson AJ. Longitudinal study of spherical refractive error in infantile nystagmus syndrome. Ophthalmic Physiol Opt. 2014; 34(3):369-75. [DOI:10.1111/opo.12117] [PMID]
- Bagheri A, Abbasi H, Tavakoli M, Sheibanizadeh A, Kheiri B, Yazdani S. Effect of rigid gas permeable contact lenses on nystagmus and visual function in hyperopic patients with infantile nystagmus syndrome. Strabismus. 2017; 25(1):17-22. [DOI:10.1080/09273972.2016.1276939] [PMID]
- Abadi R. Visual performance with contact lenses and congenital idiopathic nystagmus. Br J Physiol Opt. 1979; 33(3):32-7. [PMID]
- Abel LA. Infantile nystagmus: Current concepts in diagnosis and management. Clin Exp Optom. 2006; 89(2):57-65. [DOI:10.1111/j.1444-0938.2006.00024.x] [PMID]
- Von Noorden G. Physiology of the ocular movements, theory and management of strabismus. Binocular vision and ocular motility. Maryland Heights: Mosby; 2002. [Link]
- Barrado-Navascúes E, Vázquez-Fustes MJ, Burgos-Martínez M, Durán-Prieto E, McGauley J, Álvarez-Cerrato M. Vision evolution in the treatment of nystagmus with hydrophilic contact lenses (two clinical cases). Cont Lens Anterior Eye. 2018; 41(SUPPLEMENT 1):S70. [DOI:10.1016/j.clae.2018.03.092]
- Allen ED, Davies PD. Role of contact lenses in the management of congenital nystagmus. Br J Ophthalmol. 1983; 67(12):834-6. [DOI:10.1136/bjo.67.12.834] [PMID]
- Golubović S, Marjanović S, Cvetković D, Manić S. The application of hard contact lenses in patients with congenital nystagmus. Fortschr Ophthalmol. 1989; 86(5):535-9. [PMID]
- Jayaramachandran P, Proudlock FA, Odedra N, Gottlob I, McLean RJ. A randomized controlled trial comparing soft contact lens and rigid gas-permeable lens wearing in infantile nystagmus. Ophthalmology. 2014; 121(9):1827-36. [DOI:10.1016/j.ophtha.2014.03.007] [PMID]
- Benjamin WJ. Borish’s clinical refraction-E-Book. Amsterdam: Elsevier Health Sciences; 2006. [Link]
- Zahidi AA, Woodhouse JM, Erichsen JT, Dunn MJ. Infantile nystagmus: An optometrist’s perspective. Clin Optom (Auckl). 2017; 9:123-31. [DOI:10.2147/OPTO.S126214] [PMID]
- Hanson KS, Bedell HE, White JM, Ukwade MT. Distance and near visual acuity in infantile nystagmus. Optom Vis Sci. 2006; 83(11):823-9. [DOI:10.1097/01.opx.0000238650.33150.73] [PMID]
- Matsubayashi K, Fukushima M, Tabuchi A. Application of soft contact lenses for children with congenital nystagmus. Neuro-ophthalmology. 1992; 12(1):47-52. [DOI:10.1080/01658107.1992.11978667]
- Theodorou M, Quartilho A, Xing W, Bunce C, Rubin G, Adams G, et al. Soft contact lenses to optimize vision in adults with idiopathic infantile nystagmus: A pilot parallel randomized controlled trial. Strabismus. 2018; 26(1):11-21. [DOI:10.1080/09273972.2017.1418394] [PMID]
- Dell’Osso L, Lessell S, Van Dalen J. Nystagmus and saccadic intrusions and oscillations. Duane's clinical ophthalmology. 1988; 1:139-72. [Link]
- Cham KM, Anderson AJ, Abel LA. Task-induced stress and motivation decrease foveation-period durations in infantile nystagmus syndrome. Invest Ophthalmol Vis Sci. 2008; 49(7):2977-84. [DOI:10.1167/iovs.07-1626] [PMID]
- Catros A, Garrec A. Prisms in congenital nystagmus with compensatory deviation of the head. Bull Soc Ophtalmol Fr. 1970; 70(7):763-5. [Link]
- Rumpf RP, Tai Z, Hertle RW, Yang D. Convergence dampening of infantile nystagmus syndrome and its relationship to heterophoria. Invest Ophthalmol Vis Sci. 2009; 50(13):2838. [Link].
- Dell’Osso LF. Development of new treatments for congenital nystagmus. Ann N Y Acad Sci. 2002; 956:361-79.[DOI:10.1111/j.1749-6632.2002.tb02834.x] [PMID]
- Serra A, Dell’Osso LF, Jacobs JB, Burnstine RA. Combined gaze-angle and vergence variation in infantile nystagmus: Two therapies that improve the high-visual-acuity field and methods to measure it. Invest Ophthalmol Vis Sci. 2006; 47(6):2451-60. [DOI:10.1167/iovs.05-1320] [PMID]
- Jiménez R, Martínez-Almeida L, Salas C, Ortíz C. Contact lenses vs spectacles in myopes: Is there any difference in accommodative and binocular function? Graefes Arch Clin Exp Ophthalmol. 2011; 249(6):925-35. [DOI:10.1007/s00417-010-1570-z] [PMID]
- Lovie‐Kitchin JE, Bevanm JD, Hein B. Reading performance in children with low vision. Clin Exp Optom. 2001; 84(3):148-54. [DOI:10.1111/j.1444-0938.2001.tb04958.x] [PMID]
- Thomas MG, Gottlob I, McLean RJ, Maconachie G, Kumar A, Proudlock FA. Reading strategies in infantile nystagmus syndrome. Invest Ophthalmol Vis Sci. 2011; 52(11):8156-65. [DOI:10.1167/iovs.10-6645] [PMID]
- Woo S, Bedell HE. Beating the beat: Reading can be faster than the frequency of eye movements in persons with congenital nystagmus. Optom Vis Sci. 2006; 83(8):559-71. [DOI:10.1097/01.opx.0000230272.10471.03] [PMID]
- Barot N, McLean RJ, Gottlob I, Proudlock FA. Reading performance in infantile nystagmus. Ophthalmology. 2013; 120(6):1232-8. [DOI:10.1016/j.ophtha.2012.11.032] [PMID]
- Dysli M, Abegg M. Nystagmus does not limit reading ability in albinism. PLoS One. 2016; 11(7):e0158815. [DOI:10.1371/journal.pone.0158815] [PMID]
- McLean RJ, Windridge KC, Gottlob I. Living with nystagmus: A qualitative study. Br J Ophthalmol. 2012; 96(7):981-6. [DOI:10.1136/bjophthalmol-2011-301183] [PMID]
- Jones PH, Harris CM, Woodhouse JM, Margrain TH, Ennis FA, Erichsen JT. Stress and visual function in infantile nystagmus syndrome. Invest Ophthalmol Vis Sci. 2013; 54(13):7943-51. [DOI:10.1167/iovs.13-12560] [PMID]
- Sheth NV, Dell'Osso LF, Leigh RJ, Van Doren CL, Peckham HP. The effects of afferent stimulation on congenital nystagmus foveation periods. Vision Res. 1995; 35(16):2371-82. [PMID]
Type of Study: Review Article |
Subject:
Optometry
Received: 2024/01/31 | Accepted: 2024/02/17 | Published: 2024/06/10
Received: 2024/01/31 | Accepted: 2024/02/17 | Published: 2024/06/10