Volume 8, Issue 1 (Continuously Updated 2025)
Func Disabil J 2025, 8(1): 0-0 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Haj Ghani J, Taghizadeh G, Azad A, Habibi S A H. Predictors of Occupational Adaptation in Individuals With Parkinson’s Disease. Func Disabil J 2025; 8 (1)
URL: http://fdj.iums.ac.ir/article-1-296-en.html
URL: http://fdj.iums.ac.ir/article-1-296-en.html
1- Department of Occupational Therapy, School of Rehabilitation Sciences, Rehabilitation Research Center, Iran University of Medical Sciences, Tehran, Iran.
2- Department of Occupational Therapy, School of Rehabilitation Sciences, Rehabilitation Research Center, Iran University of Medical Sciences, Tehran, Iran. ,taghizadeh.gh@iums.ac.ir
3- Department of Neurology, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
2- Department of Occupational Therapy, School of Rehabilitation Sciences, Rehabilitation Research Center, Iran University of Medical Sciences, Tehran, Iran. ,
3- Department of Neurology, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
Full-Text [PDF 534 kb]
(329 Downloads)
| Abstract (HTML) (844 Views)
Full-Text: (216 Views)
Introduction
Occupational adaptation (OA) is a dynamic and ongoing process through which individuals modifies their participation in meaningful activities to address challenges arising from changes in their abilities, environments, or life circumstances [1]. This adaptive process holds particular significance for individuals living in chronic and progressive conditions, such as Parkinson’s disease (PD). Parkinson’s disease is a neurodegenerative disorder characterized by both motor and non-motor symptoms, presenting persistent challenges that can profoundly affect an individual’s ability to develop OA [2, 3].
Promoting OA is essential because it empowers individuals to preserve their independence by improving participation and well-being through continued engagement in meaningful daily activities.
The occupational adaptation model highlights relative mastery as a primary indicator of OA, including effectiveness, efficiency, and satisfaction [1]. The relative mastery scale (RMS) is a validated instrument for measuring relative mastery. It employed a 5-point Likert scale in conjunction with open-ended questions focusing on selected occupations [4]. The application of RMS enables clinicians and researchers to comprehensively understand an individual’s adaptive response to occupational challenges.
Literature proposes a longitudinal relationship between sleep quality, depression, and fatigue in the PD population. Zhou et al. reported that PD patients with fatigue exhibit more motor and non-motor symptoms (i.e. sleep, depression, and cognitive impairment) [5]. Moreover, psychological factors, such as depression and apathy, share a linear worsening trajectory [6, 7]. Patients with depression experience substantial disease progression, cognitive decline, and restricted participation in activities of daily living for 12 months [8]. Furthermore, a substantial increase in the fear of falling over longitudinal follow-up leads to difficulties in occupational participation [9].
This study investigated psychological predictors of OA in individuals with PD. We hypothesized that specific prevalent demographic and psychological symptoms in PD patients, which are most related to their quality of life, are predictors of OA scores [10-13]. Therefore, we hypothesized that some demographic and prevalent clinical variables in PD would serve as predictors of OA scores. By identifying these predictive elements, this study aimed to provide valuable insights that can inform targeted interventions, ultimately supporting OA and enhancing the participation of those living with PD.
Materials and Methods
A convenient non-probability sampling method was employed to recruit 100 individuals diagnosed with PD from neurological outpatient centers in Tehran City, Iran. The inclusion criteria included a diagnosis based on the UK Brain Bank criteria [14], demonstrating no significant cognitive impairments (as assessed by a Montreal cognitive assessment score over 24) [15], and possessing proficiency in reading and writing in Persian. The exclusion criteria included individuals with comorbid neurological conditions, and participants were given the option to withdraw from the study at any time if they expressed reluctance to continue participating.
Procedure
Demographic characteristics, including age, sex, time since PD diagnosis, Levodopa equivalent daily dose (referring to the dosing schedule) [16], Hoehn and Yahr staging scale, and living arrangements, were assessed by an occupational therapist, with breaks offered as needed. The Lille apathy rating scale (LARS) was administered. Other surveys were provided to participants in a packet, including the RMS, hospital anxiety and depression scale-depression subscale (HADS-D), Tilburg frailty indicator (TFI), Pittsburgh sleep quality index (PSQI), falls efficacy scale-international (FES-I), fatigue severity scale (FSS), and Parkinson disease questionnaire-39 (PDQ-39).
Patients were required to complete the questionnaires in their “ON” state, 1 h after levodopa intake. Participants were given 48 to 72 h to complete the questionnaires and were encouraged to highlight any concerns. At the subsequent meeting for survey collection, we addressed any issues that arose and ensured clarity and completion of all questionnaire sections.
Assessments
The relative mastery scale (RMS) was developed to assess the concept of OA in occupational activities chosen by clients. It comprises six items, each rated on a scale ranging from -2 to +2. The cumulative score on this scale ranges from -12 to +12, with higher scores indicating more significant OA. In addition to these items, the scale included three open-ended questions on performance in the selected occupational activity. The validity and reliability of the scale have been established, with a reported Cronbach’s α value of 0.94 [17]. The translation of the RMS into Persian has been completed for caregivers of patients with multiple sclerosis [18]. The research team assessed this test’s reliability in people with PD, and Cronbach’s α was 0.87. However, these results have not been reported.
The Lille apathy rating scale (LARS) measures apathy with a semi-structured interview in nine domains. The total score ranges from -15 to +15, with higher scores indicating a higher level of apathy. The minimal clinically important difference (MCID) was 2.99 points. The LARS has been validated in a PD population and used in Persian populations [7, 19].
The hospital anxiety and depression scale-depression subscale (HADS-D) measures depression and anxiety with two 7-item subscales. Each item is scored from 0 (no problem) to 3 (severe problem). The MCID for depression subscale score of this scale was 1.7 points. This scale has been extensively used in the PD population and has a Cronbach’s α (0.86, for the depression subscale) in Persian [20, 21].
The Tilburg frailty indicator (TFI) is a self-report questionnaire designed to measure frailty in older adults. It consists of 15 items that assess three domains: Physical, psychological, and social. Each item is scored on a 3-point scale (0=no problem to 2=severe problem). The MCID for the total score on this scale is one point. The TFI demonstrated good reliability in patients with PD and acceptable psychometric properties in Persian patients [22, 23].
The Pittsburgh sleep quality index (PSQI) evaluates sleep quality with 24 items. Items are scored from zero to three, with higher scores indicating poorer subjective sleep quality. The MCID for this scale is three points. Farrahi Moghaddam et al. reported 0.78 of Cronbach’s α for this scale in healthy people [24].
The falls efficacy scale-international (FES-I) evaluates fall concerns in various physical and social activities with 16 items. Items are rated on a 4-point Likert scale (1=not concerned; 4=very concerned). The total score ranged from 16 to 64, with higher scores denoting a higher fear of falling. The MCID for the total score on this scale was four points. Psychometric properties were evaluated in patients with PD, and Cronbach’s α for the Persian version was 0.94 [25, 26].
The fatigue severity scale (FSS) measures the severity of fatigue and its effect on daily life activities. This scale consists of nine items, ranging from 1 (strongly disagree) to 7 (strongly agree). The total score was calculated by summing the item scores divided by nine. Higher scores reflect higher fatigue levels. The MCID for this scale was 0.45. This scale has been used in the PD population and has an interclass correlation (ICC) of 0.93 in Iranian people [27, 28].
The Parkinson disease questionnaire-39 (PDQ-39) is a tool designed to measure the quality of life in individuals with PD. It assesses how the disease impacts daily living and well-being. The PDQ-39 includes 39 items divided into eight dimensions: Mobility, activities of daily living, emotional well-being, stigma, social support, cognition, communication, and bodily discomfort. The total score ranged from 0 to 100, with higher scores indicating worse conditions. The MCID of the total score was 1.6. This questionnaire showed acceptable validation properties in Iranian with PD [29].
Statistical analysis
The Shapiro-Wilk test was applied to evaluate the normal distribution, and descriptive statistics were used to summarize demographic and clinical characteristics. Statistical significance was set at 0.05. A stepwise regression model was employed, using correlated demographic and clinical variables (Hoehn and Yahr stages, disease duration, apathy, frailty, fatigue, fall concern, sleep quality, quality of life, and depression) as independent variables and the RMS total score as the dependent variable. All the variables were included simultaneously in the regression equation.
Results
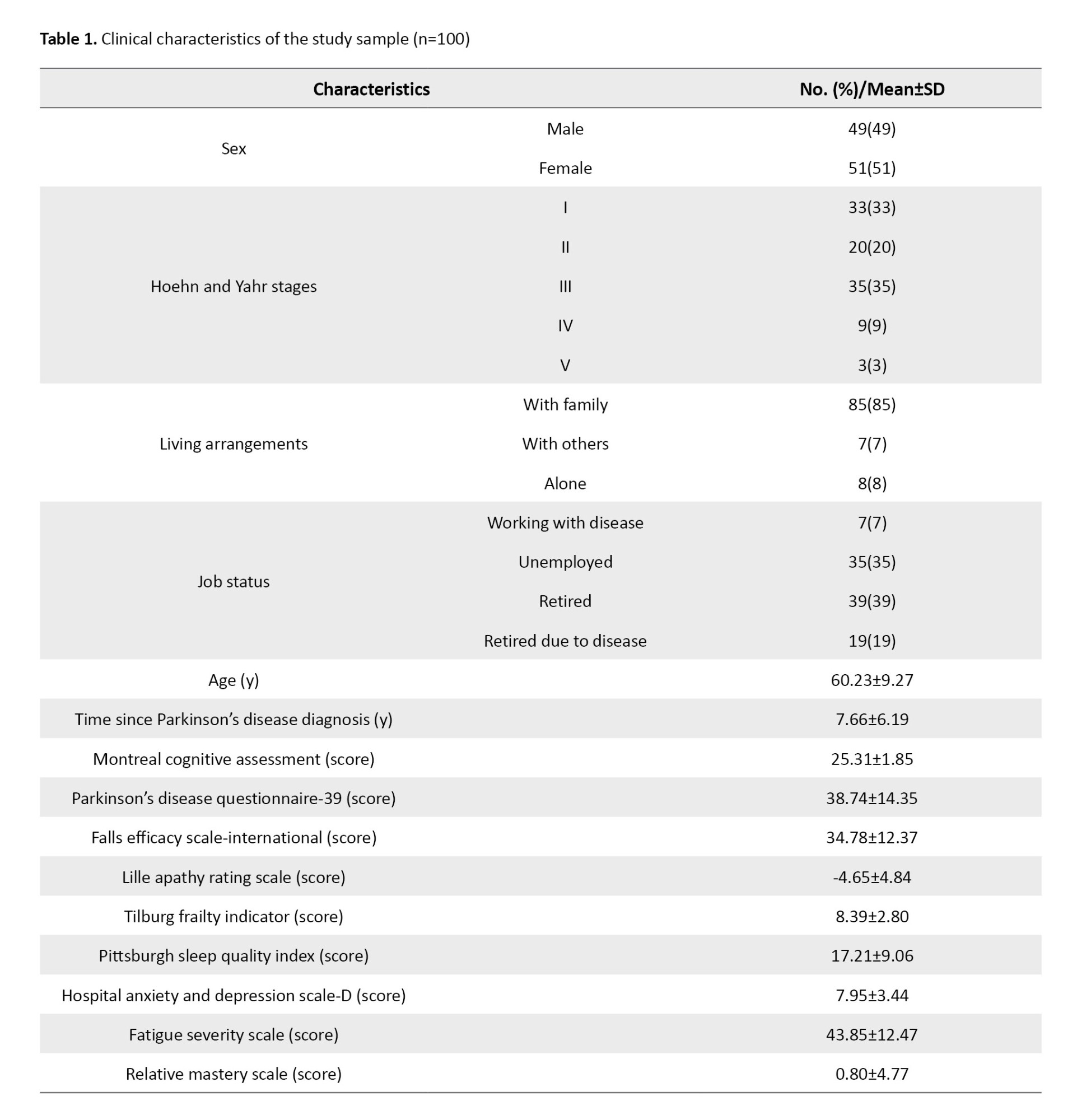
A total of 100 individuals diagnosed with PD participated in this study. The participants had a mean age of 60.23 years, with a standard deviation of 9.27 years. The average duration of the disease since diagnosis was 7.66 years, accompanied by a standard deviation of 5.19 years. Table 1 presents detailed demographic information.
The RMS exhibited a significant (P<0.05) moderate correlation with PDQ-39 (P=-0.42), hospital anxiety and depression scale-depression subscale (HADS-D) (P=-0.37), TFI (P=-0.42), FESI (P=-0.37), and Pittsburgh sleep quality index (PSQI) (P=-0.32). Additionally, a weak correlation was observed with LARS (P=-0.29), disease duration (P=-0.20), and H and Y stage (P=-0.29). No significant correlation was observed between RMS and other explored demographic variables.
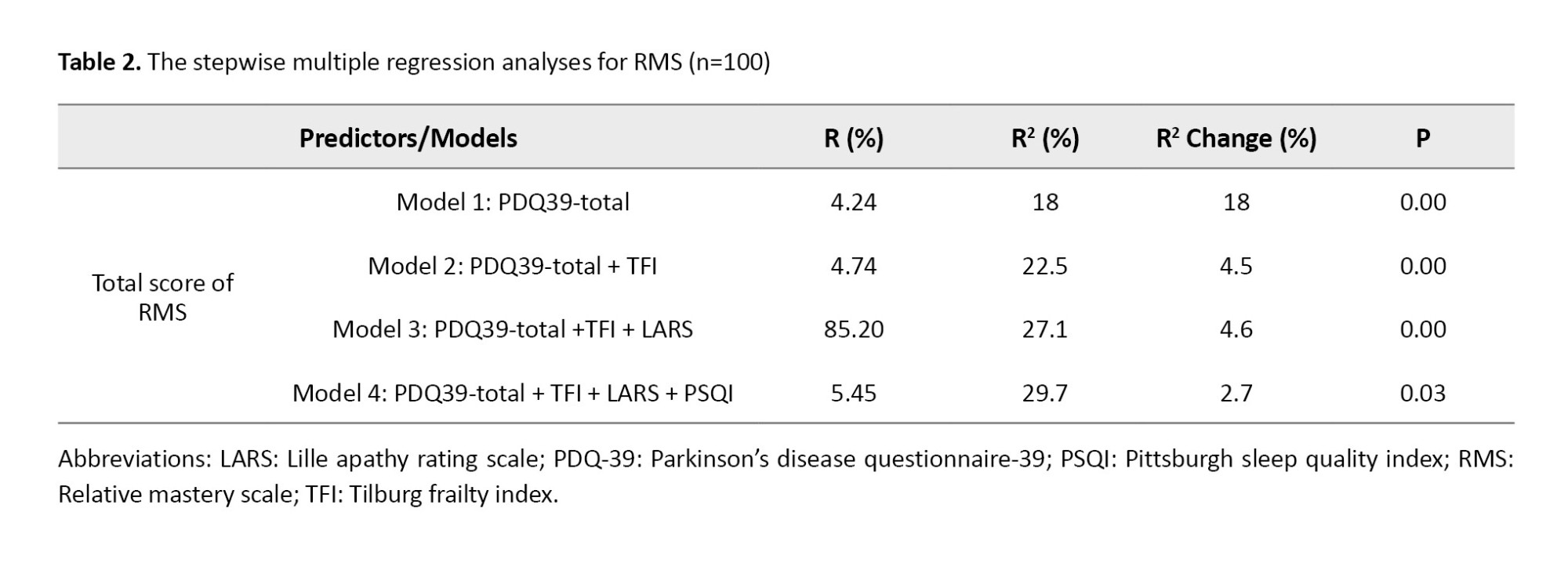
A comprehensive standard multiple regression analysis was conducted to predict OA using RMS scores. Various demographic and clinical variables were simultaneously included in the model to ensure a thorough examination of contributing factors. The analysis revealed a significant model, explaining 29.7% of the variance in RMS scores. It indicated that quality of life, frailty, apathy, and sleep quality were significant predictors of the total RMS score (P<0.05). Table 2 presents the results of the stepwise analysis.
These results highlight the multifaceted nature of occupational adaptation in individuals with PD and the importance of addressing these critical factors in clinical practice. The practical implications of these findings are substantial, and advocate a more comprehensive approach to patient care.
Discussion
Occupational adaptation is a learning process that enables individuals to develop, modify, or acquire the skills necessary to remain engaged in their desired occupations [1]. This process is particularly crucial for those dealing with chronic conditions or disabilities, such as PD. Given the essential role of OA in rehabilitation and ongoing management of PD as a degenerative condition, the ability to predict the state of OA is crucial. This study found that quality of life, frailty, apathy, and sleep quality were the leading factors that predicted relative mastery.
Stepwise regression analysis revealed that quality of life, frailty, apathy, and sleep quality were the most significant predictors of the RMS total score among patients with PD. Quality of life, the strongest predictor of OA, is a multidimensional construct encompassing various aspects of an individual’s life. These aspects include physical, psychological, and social components, and subjective life satisfaction [24]. OA involves interaction between people, their environments, and their occupations. As a comprehensive concept, quality of life incorporates all these elements.
In the context of frailty, higher levels of perceived mastery can empower older adults to better cope with their health challenges, potentially mitigating the adverse effects of frailty. Studies have indicated that older adults who perceive themselves as having more excellent mastery report better quality of life and resilience to challenges associated with frailty [25].
Apathy was another significant predictor RM. This often results in feelings of disconnection and decreases motivation for goal-directed behaviors. Individuals with apathy may not pay close attention to the outcomes of their actions or lack the desire to learn from their experiences [13]. Conversely, engaging in daily activities is essential to develop a sense of mastery in life. Since apathy typically manifests as a lack of interest or motivation to participate in these activities, fostering a sense of mastery may encourage individuals to become more involved and address their symptoms of apathy.
Poor sleep quality is a prevalent symptom of PD, which may be due to disruption of sleep-regulating pathways in this population [26]. Previous studies have demonstrated a strong correlation between poor sleep quality and disruptions in advanced cognitive processes, directly impacting the essential sub-processes for learning and fostering mastery in these individuals [27, 28].
Conclusion
The results of this study have significant implications for assessing and managing occupational adaptation in individuals with PD. This research underscores the importance of quality of life, frailty, apathy, and sleep quality as critical predictors of occupational adaptation, with quality of life emerging as the most influential factor. This understanding equips healthcare professionals, researchers, and students with knowledge of the key elements that influence occupational adaptation and their role in applying it in their work. These results have the potential to influence the field significantly, inspiring the development of more effective interventions and strategies, thereby advancing the field and improving the well-being of patients with PD.
This study has several significant limitations. First, convenience sampling was used. While this approach allowed for the inclusion of a diverse range of participants, it also meant that the study selected readily available participants, which may limit how well the results apply to all patients with PD.
This study’s focus on individuals with good cognitive health is a significant limitation. This approach may exclude patients with cognitive decline, a common feature of PD, potentially leading to findings that do not fully represent the population with PD. Furthermore, the limited number of participants in advanced stages of the disease, a crucial group for a comprehensive understanding of PD, is an area that future research should address. Another issue that warrants attention is the nearly equal gender distribution among participants. Given that PD is more prevalent in men, this could introduce a bias in the findings. Moreover, higher levels of apathy experienced by men could potentially reduce their participation. This sex imbalance underscores the need for further research to validate the conclusions in the entire population affected by PD.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Iran University of Medical Sciences, Tehran, Iran (Code: IR.IUMS.REC.1402.101). Participants signed a form to provide informed consent. Each participant was assigned a unique code to ensure privacy and anonymity during the research.
Funding
This article extracted from the master thesis of Jafar Haj Ghani, approved by Iran University of Medical Sciences, Tehran, Iran.
Authors' contributions
Conceptualization and supervision: Ghorban Taghizadeh, Akram Azad, Seyed Amir Hasan Habibi; Data analysis: Ghorban Taghizadeh; Sample recruitment, data collection, writing article: Jafar Haj Ghani.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to acknowledge Iran University of Medical Science for providing the facilities and resources necessary for this research.
References
Occupational adaptation (OA) is a dynamic and ongoing process through which individuals modifies their participation in meaningful activities to address challenges arising from changes in their abilities, environments, or life circumstances [1]. This adaptive process holds particular significance for individuals living in chronic and progressive conditions, such as Parkinson’s disease (PD). Parkinson’s disease is a neurodegenerative disorder characterized by both motor and non-motor symptoms, presenting persistent challenges that can profoundly affect an individual’s ability to develop OA [2, 3].
Promoting OA is essential because it empowers individuals to preserve their independence by improving participation and well-being through continued engagement in meaningful daily activities.
The occupational adaptation model highlights relative mastery as a primary indicator of OA, including effectiveness, efficiency, and satisfaction [1]. The relative mastery scale (RMS) is a validated instrument for measuring relative mastery. It employed a 5-point Likert scale in conjunction with open-ended questions focusing on selected occupations [4]. The application of RMS enables clinicians and researchers to comprehensively understand an individual’s adaptive response to occupational challenges.
Literature proposes a longitudinal relationship between sleep quality, depression, and fatigue in the PD population. Zhou et al. reported that PD patients with fatigue exhibit more motor and non-motor symptoms (i.e. sleep, depression, and cognitive impairment) [5]. Moreover, psychological factors, such as depression and apathy, share a linear worsening trajectory [6, 7]. Patients with depression experience substantial disease progression, cognitive decline, and restricted participation in activities of daily living for 12 months [8]. Furthermore, a substantial increase in the fear of falling over longitudinal follow-up leads to difficulties in occupational participation [9].
This study investigated psychological predictors of OA in individuals with PD. We hypothesized that specific prevalent demographic and psychological symptoms in PD patients, which are most related to their quality of life, are predictors of OA scores [10-13]. Therefore, we hypothesized that some demographic and prevalent clinical variables in PD would serve as predictors of OA scores. By identifying these predictive elements, this study aimed to provide valuable insights that can inform targeted interventions, ultimately supporting OA and enhancing the participation of those living with PD.
Materials and Methods
A convenient non-probability sampling method was employed to recruit 100 individuals diagnosed with PD from neurological outpatient centers in Tehran City, Iran. The inclusion criteria included a diagnosis based on the UK Brain Bank criteria [14], demonstrating no significant cognitive impairments (as assessed by a Montreal cognitive assessment score over 24) [15], and possessing proficiency in reading and writing in Persian. The exclusion criteria included individuals with comorbid neurological conditions, and participants were given the option to withdraw from the study at any time if they expressed reluctance to continue participating.
Procedure
Demographic characteristics, including age, sex, time since PD diagnosis, Levodopa equivalent daily dose (referring to the dosing schedule) [16], Hoehn and Yahr staging scale, and living arrangements, were assessed by an occupational therapist, with breaks offered as needed. The Lille apathy rating scale (LARS) was administered. Other surveys were provided to participants in a packet, including the RMS, hospital anxiety and depression scale-depression subscale (HADS-D), Tilburg frailty indicator (TFI), Pittsburgh sleep quality index (PSQI), falls efficacy scale-international (FES-I), fatigue severity scale (FSS), and Parkinson disease questionnaire-39 (PDQ-39).
Patients were required to complete the questionnaires in their “ON” state, 1 h after levodopa intake. Participants were given 48 to 72 h to complete the questionnaires and were encouraged to highlight any concerns. At the subsequent meeting for survey collection, we addressed any issues that arose and ensured clarity and completion of all questionnaire sections.
Assessments
The relative mastery scale (RMS) was developed to assess the concept of OA in occupational activities chosen by clients. It comprises six items, each rated on a scale ranging from -2 to +2. The cumulative score on this scale ranges from -12 to +12, with higher scores indicating more significant OA. In addition to these items, the scale included three open-ended questions on performance in the selected occupational activity. The validity and reliability of the scale have been established, with a reported Cronbach’s α value of 0.94 [17]. The translation of the RMS into Persian has been completed for caregivers of patients with multiple sclerosis [18]. The research team assessed this test’s reliability in people with PD, and Cronbach’s α was 0.87. However, these results have not been reported.
The Lille apathy rating scale (LARS) measures apathy with a semi-structured interview in nine domains. The total score ranges from -15 to +15, with higher scores indicating a higher level of apathy. The minimal clinically important difference (MCID) was 2.99 points. The LARS has been validated in a PD population and used in Persian populations [7, 19].
The hospital anxiety and depression scale-depression subscale (HADS-D) measures depression and anxiety with two 7-item subscales. Each item is scored from 0 (no problem) to 3 (severe problem). The MCID for depression subscale score of this scale was 1.7 points. This scale has been extensively used in the PD population and has a Cronbach’s α (0.86, for the depression subscale) in Persian [20, 21].
The Tilburg frailty indicator (TFI) is a self-report questionnaire designed to measure frailty in older adults. It consists of 15 items that assess three domains: Physical, psychological, and social. Each item is scored on a 3-point scale (0=no problem to 2=severe problem). The MCID for the total score on this scale is one point. The TFI demonstrated good reliability in patients with PD and acceptable psychometric properties in Persian patients [22, 23].
The Pittsburgh sleep quality index (PSQI) evaluates sleep quality with 24 items. Items are scored from zero to three, with higher scores indicating poorer subjective sleep quality. The MCID for this scale is three points. Farrahi Moghaddam et al. reported 0.78 of Cronbach’s α for this scale in healthy people [24].
The falls efficacy scale-international (FES-I) evaluates fall concerns in various physical and social activities with 16 items. Items are rated on a 4-point Likert scale (1=not concerned; 4=very concerned). The total score ranged from 16 to 64, with higher scores denoting a higher fear of falling. The MCID for the total score on this scale was four points. Psychometric properties were evaluated in patients with PD, and Cronbach’s α for the Persian version was 0.94 [25, 26].
The fatigue severity scale (FSS) measures the severity of fatigue and its effect on daily life activities. This scale consists of nine items, ranging from 1 (strongly disagree) to 7 (strongly agree). The total score was calculated by summing the item scores divided by nine. Higher scores reflect higher fatigue levels. The MCID for this scale was 0.45. This scale has been used in the PD population and has an interclass correlation (ICC) of 0.93 in Iranian people [27, 28].
The Parkinson disease questionnaire-39 (PDQ-39) is a tool designed to measure the quality of life in individuals with PD. It assesses how the disease impacts daily living and well-being. The PDQ-39 includes 39 items divided into eight dimensions: Mobility, activities of daily living, emotional well-being, stigma, social support, cognition, communication, and bodily discomfort. The total score ranged from 0 to 100, with higher scores indicating worse conditions. The MCID of the total score was 1.6. This questionnaire showed acceptable validation properties in Iranian with PD [29].
Statistical analysis
The Shapiro-Wilk test was applied to evaluate the normal distribution, and descriptive statistics were used to summarize demographic and clinical characteristics. Statistical significance was set at 0.05. A stepwise regression model was employed, using correlated demographic and clinical variables (Hoehn and Yahr stages, disease duration, apathy, frailty, fatigue, fall concern, sleep quality, quality of life, and depression) as independent variables and the RMS total score as the dependent variable. All the variables were included simultaneously in the regression equation.
Results
A total of 100 individuals diagnosed with PD participated in this study. The participants had a mean age of 60.23 years, with a standard deviation of 9.27 years. The average duration of the disease since diagnosis was 7.66 years, accompanied by a standard deviation of 5.19 years. Table 1 presents detailed demographic information.

The RMS exhibited a significant (P<0.05) moderate correlation with PDQ-39 (P=-0.42), hospital anxiety and depression scale-depression subscale (HADS-D) (P=-0.37), TFI (P=-0.42), FESI (P=-0.37), and Pittsburgh sleep quality index (PSQI) (P=-0.32). Additionally, a weak correlation was observed with LARS (P=-0.29), disease duration (P=-0.20), and H and Y stage (P=-0.29). No significant correlation was observed between RMS and other explored demographic variables.
A comprehensive standard multiple regression analysis was conducted to predict OA using RMS scores. Various demographic and clinical variables were simultaneously included in the model to ensure a thorough examination of contributing factors. The analysis revealed a significant model, explaining 29.7% of the variance in RMS scores. It indicated that quality of life, frailty, apathy, and sleep quality were significant predictors of the total RMS score (P<0.05). Table 2 presents the results of the stepwise analysis.

These results highlight the multifaceted nature of occupational adaptation in individuals with PD and the importance of addressing these critical factors in clinical practice. The practical implications of these findings are substantial, and advocate a more comprehensive approach to patient care.
Discussion
Occupational adaptation is a learning process that enables individuals to develop, modify, or acquire the skills necessary to remain engaged in their desired occupations [1]. This process is particularly crucial for those dealing with chronic conditions or disabilities, such as PD. Given the essential role of OA in rehabilitation and ongoing management of PD as a degenerative condition, the ability to predict the state of OA is crucial. This study found that quality of life, frailty, apathy, and sleep quality were the leading factors that predicted relative mastery.
Stepwise regression analysis revealed that quality of life, frailty, apathy, and sleep quality were the most significant predictors of the RMS total score among patients with PD. Quality of life, the strongest predictor of OA, is a multidimensional construct encompassing various aspects of an individual’s life. These aspects include physical, psychological, and social components, and subjective life satisfaction [24]. OA involves interaction between people, their environments, and their occupations. As a comprehensive concept, quality of life incorporates all these elements.
In the context of frailty, higher levels of perceived mastery can empower older adults to better cope with their health challenges, potentially mitigating the adverse effects of frailty. Studies have indicated that older adults who perceive themselves as having more excellent mastery report better quality of life and resilience to challenges associated with frailty [25].
Apathy was another significant predictor RM. This often results in feelings of disconnection and decreases motivation for goal-directed behaviors. Individuals with apathy may not pay close attention to the outcomes of their actions or lack the desire to learn from their experiences [13]. Conversely, engaging in daily activities is essential to develop a sense of mastery in life. Since apathy typically manifests as a lack of interest or motivation to participate in these activities, fostering a sense of mastery may encourage individuals to become more involved and address their symptoms of apathy.
Poor sleep quality is a prevalent symptom of PD, which may be due to disruption of sleep-regulating pathways in this population [26]. Previous studies have demonstrated a strong correlation between poor sleep quality and disruptions in advanced cognitive processes, directly impacting the essential sub-processes for learning and fostering mastery in these individuals [27, 28].
Conclusion
The results of this study have significant implications for assessing and managing occupational adaptation in individuals with PD. This research underscores the importance of quality of life, frailty, apathy, and sleep quality as critical predictors of occupational adaptation, with quality of life emerging as the most influential factor. This understanding equips healthcare professionals, researchers, and students with knowledge of the key elements that influence occupational adaptation and their role in applying it in their work. These results have the potential to influence the field significantly, inspiring the development of more effective interventions and strategies, thereby advancing the field and improving the well-being of patients with PD.
This study has several significant limitations. First, convenience sampling was used. While this approach allowed for the inclusion of a diverse range of participants, it also meant that the study selected readily available participants, which may limit how well the results apply to all patients with PD.
This study’s focus on individuals with good cognitive health is a significant limitation. This approach may exclude patients with cognitive decline, a common feature of PD, potentially leading to findings that do not fully represent the population with PD. Furthermore, the limited number of participants in advanced stages of the disease, a crucial group for a comprehensive understanding of PD, is an area that future research should address. Another issue that warrants attention is the nearly equal gender distribution among participants. Given that PD is more prevalent in men, this could introduce a bias in the findings. Moreover, higher levels of apathy experienced by men could potentially reduce their participation. This sex imbalance underscores the need for further research to validate the conclusions in the entire population affected by PD.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Iran University of Medical Sciences, Tehran, Iran (Code: IR.IUMS.REC.1402.101). Participants signed a form to provide informed consent. Each participant was assigned a unique code to ensure privacy and anonymity during the research.
Funding
This article extracted from the master thesis of Jafar Haj Ghani, approved by Iran University of Medical Sciences, Tehran, Iran.
Authors' contributions
Conceptualization and supervision: Ghorban Taghizadeh, Akram Azad, Seyed Amir Hasan Habibi; Data analysis: Ghorban Taghizadeh; Sample recruitment, data collection, writing article: Jafar Haj Ghani.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to acknowledge Iran University of Medical Science for providing the facilities and resources necessary for this research.
References
- Schkade JK, Schultz S. Occupational adaptation: toward a holistic approach for contemporary practice, Part 1. Am J Occup Ther. 1992; 46(9):829-37. [DOI:10.5014/ajot.46.9.829] [PMID]
- Dibble LE, Cavanaugh JT, Earhart GM, Ellis TD, Ford MP, Foreman KB. Charting the progression of disability in Parkinson disease: Study protocol for a prospective longitudinal cohort study. BMC Neurol. 2010; 10:110. [DOI:10.1186/1471-2377-10-110] [PMID]
- Duncan RP, Earhart GM. Measuring participation in individuals with Parkinson disease: Relationships with disease severity, quality of life, and mobility. Disabil Rehabil. 2011; 33(15-16):1440-6. [DOI:10.3109/09638288.2010.533245] [PMID]
- George-Paschal L, Krusen NE. Evaluating psychometric properties of the relative Mastery Scale: An occupational adaptation instrument. Am J Occup Ther 2023; 77(2). [DOI:10.5014/ajot.2023.77S2-PO28]
- Zhou X, Xiang Y, Song T, Zhao Y, Pan H, Xu Q, et al. Characteristics of fatigue in Parkinson's disease: A longitudinal cohort study. Front Aging Neurosci. 2023; 15:1133705. [DOI:10.3389/fnagi.2023.1133705] [PMID]
- Lien WH, Lien WC, Kuan TS, Wu ST, Chen YT, Chiu CJ. Parkinson disease and musculoskeletal pain: an 8-year population-based cohort study. Pain. 2017; 158(7):1234-40. [DOI:10.1097/j.pain.0000000000000904] [PMID]
- Zahodne LB, Young S, Kirsch‐Darrow L, Nisenzon A, Fernandez HH, Okun MS, et al. Examination of the Lille apathy rating scale in Parkinson disease. Mov Disord. 2009; 24(5):677-83. [DOI:10.1002/mds.22441] [PMID]
- Starkstein SE, Mayberg HS, Leiguarda R, Preziosi TJ, Robinson RG. A prospective longitudinal study of depression, cognitive decline, and physical impairments in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 1992; 55(5):377-82. [DOI:10.1136/jnnp.55.5.377] [PMID]
- Lindh-Rengifo M, Jonasson SB, Mattsson N, Ullén S, Nilsson MH. Predictive factors of concerns about falling in people with parkinson's disease: A 3-year longitudinal study. Parkinsons Dis. 2019; 2019:4747320. [DOI:10.1155/2019/4747320] [PMID]
- Rotaru L, Gavriliuc O, Grosu O. Depression in patients with Parkinson’s disease. Preliminary results of the cohort study. Bull Acad Sci Moldova. Med Sci. 2022; 74(3):91-4. [DOI:10.52692/1857-0011.2022.3-74.16]
- Han IT, Ha CK, Hong CG, Choi JY, Ahn JH, Park JJ, et al. Behavioral and psychological symptoms in patients with Parkinson’s disease according to cognitive function. Dement Neurocogn Disord. 2012; 11(3):104-10. [DOI:10.12779/dnd.2012.11.3.104]
- Rahman S, Griffin H, Quinn N, Jahanshahi M. On the nature of fear of falling in Parkinson’s disease. Behav Neurol. 2011; 24(3):219-28. [DOI: 10.3233/BEN-2011-0330] [PMID]
- Shafazand S, Wallace DM, Arheart KL, Vargas S, Luca CC, Moore H, et al. Insomnia, sleep quality, and quality of life in mild to moderate Parkinson's Disease. Ann Am Thorac Soc. 2017; 14(3):412-9. [DOI:10.1513/AnnalsATS.201608-625OC] [PMID]
- Daniel SE, Lees AJ. Parkinson's Disease society brain bank, London: Overview and research. J Neural Transm Suppl. 1993; 39:165-72. [PMID]
- Foroughan M, Jafari Z, Shirin Bayan P, Ghaem Magham Farahani Z, Rahgozar M. [Validation of mini-mental state examination (MMSE) in the elderly population of Tehran (Persian)]. Adv Cogn Sci. 2008; 10(2):29-37. [Link]
- Razmy A, Lang AE, Shapiro CM. Predictors of impaired daytime sleep and wakefulness in patients with Parkinson disease treated with older (ergot) vs newer (nonergot) dopamine agonists. Arch Neurol. 2004; 61(1):97-102. [DOI:10.1001/archneur.61.1.97] [PMID]
- George-Paschal L, Krusen NE, Fan CW. Psychometric evaluation of the Relative Mastery Scale: An occupational adaptation instrument. OTJR (Thorofare N J). 2022; 42(2):154-61. [DOI:10.1177/15394492211060877] [PMID]
- Hassani Mehraban A, Motaharinezhad F, Ghahari S, Lajevardi L, Mohebbirad M. Psychometric evaluation and feasibility of the Persian-Relative Mastery Scale in the caregivers. Br J Occup Ther. 2024; 87(8):504-11. [DOI:10.1177/03080226241246137]
- Jamali A, Baluchnejadmojarad T, Jazaeri SZ, Abedi S, Mehdizadeh H, Taghizadeh G. Lille Apathy Rating Scale-Patient Version in Stroke Survivors: Psychometric properties and diagnostic accuracy. J Am Med Dir Assoc. 2024; 25(10):105193. [DOI:10.1016/j.jamda.2024.105193] [PMID]
- Rodriguez-Blazquez C, Frades-Payo B, Forjaz MJ, de Pedro-Cuesta J, Martinez-Martin P; Longitudinal Parkinson's Disease Patient Study Group. Psychometric attributes of the Hospital Anxiety and Depression Scale in Parkinson's disease. Mov Disord. 2009; 24(4):519-25. [DOI:10.1002/mds.22321] [PMID]
- Montazeri A, Vahdaninia M, Ebrahimi M, Jarvandi S. The Hospital Anxiety and Depression Scale (HADS): Translation and validation study of the Iranian version. Health Qual Life Outcomes. 2003; 1:14. [DOI:10.1186/1477-7525-1-14] [PMID]
- Roland KP, Cornett KM, Theou O, Jakobi JM, Jones GR. Concurrence of Frailty and Parkinson's Disease. J Frailty Aging. 2012; 1(3):123-7. [DOI:10.14283/jfa.2012.20] [PMID]
- Safarnavadeh M, Salehi L. Psychometric adequacy of the persian adapted version of the tilburg frailty indicator (P-TFI). BMC Geriatr. 2024; 24(1):623. [PMID]
- Farrahi Moghaddam J, Nakhaee N, Sheibani V, Garrusi B, Amirkafi A. Reliability and validity of the Persian version of the Pittsburgh Sleep Quality Index (PSQI-P). Sleep Breath. 2012; 16(1):79-82. [DOI:10.1007/s11325-010-0478-5] [PMID]
- Norouzi Z, Ghoochani BZ, Kaveh MH, Sokout T, Asadollahi A, Abyad A. Psychometric Properties of the Falls Efficacy Scale-International, cut-off points, and validating its short version among Iranian Older People. Oman Med J. 2023; 38(1):e460. [DOI:10.5001/omj.2023.39] [PMID]
- Jonasson SB, Nilsson MH, Lexell J. Psychometric properties of the original and short versions of the Falls Efficacy Scale-International (FES-I) in people with Parkinson's disease. Health Qual Life Outcomes. 2017; 15(1):116. [DOI:10.1186/s12955-017-0689-6] [PMID]
- Herlofson K, Larsen JP. Measuring fatigue in patients with Parkinson’s disease-the fatigue severity scale. Eur J Neurol. 2002; 9(6):595-600. [DOI:10.1046/j.1468-1331.2002.00444.x] [PMID]
- Shahvaroughi FA, Azimian M, Falahpour M, Karimlou M. Fatigue severity scale (FSS): Evaluatiopn of reliability of the Persian version among persons with multiple sclerosis. J Rehabil. 2010; 10(4):46-51. [Link]
- Dehghan A, Ghaem H, Borhani A, Safari R, Moosazadeh M, Gholami A. Evaluation of reliability and validity of PDQ-39: Questionnaire in Iranian patients with Parkinson’s disease. Zahedan J Res Med Sci. 2016; 18(3):e6245. [DOI:10.17795/zjrms-6245]
Type of Study: Research |
Subject:
Occupational Therapy
Received: 2024/11/26 | Accepted: 2024/12/30 | Published: 2025/03/2
Received: 2024/11/26 | Accepted: 2024/12/30 | Published: 2025/03/2








