Volume 8, Issue 1 (Continuously Updated 2025)
Func Disabil J 2025, 8(1): 0-0 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Banimahdi R, Akhavan Hejazi S M, Hosseini S A, Akbarfahimi N, Vahedi M. Psychometric Properties of the Persian Version of the Frontal Assessment Battery in Patients With Traumatic Brain Injury. Func Disabil J 2025; 8 (1)
URL: http://fdj.iums.ac.ir/article-1-287-en.html
URL: http://fdj.iums.ac.ir/article-1-287-en.html
Ramin Banimahdi1 

 , Seyed Majid Akhavan Hejazi2
, Seyed Majid Akhavan Hejazi2 

 , Seyed Ali Hosseini3
, Seyed Ali Hosseini3 

 , Nazila Akbarfahimi *4
, Nazila Akbarfahimi *4 

 , Mohsen Vahedi5
, Mohsen Vahedi5 




 , Seyed Majid Akhavan Hejazi2
, Seyed Majid Akhavan Hejazi2 

 , Seyed Ali Hosseini3
, Seyed Ali Hosseini3 

 , Nazila Akbarfahimi *4
, Nazila Akbarfahimi *4 

 , Mohsen Vahedi5
, Mohsen Vahedi5 


1- School of Rehabilitation Sciences, Faculty of Health Sciences, University of Ottawa, Ottawa, Canada.
2- Brain and Spinal Cord Injuries Ward, Rofeideh Rehabilitation Hospital, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
3- Department of Occupational Therapy, School of Rehabilitation Sciences, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
4- Department of Occupational Therapy, School of Rehabilitation Sciences, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran. ,na.akbarfahimi@uswr.ac.ir
5- Department of Biostatistics and Epidemiology, School of Rehabilitation Sciences, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
2- Brain and Spinal Cord Injuries Ward, Rofeideh Rehabilitation Hospital, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
3- Department of Occupational Therapy, School of Rehabilitation Sciences, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
4- Department of Occupational Therapy, School of Rehabilitation Sciences, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran. ,
5- Department of Biostatistics and Epidemiology, School of Rehabilitation Sciences, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
Keywords: Traumatic brain injury (TBI), Frontal assessment battery (FAB), Validity, Reliability, Cut-off
Full-Text [PDF 677 kb]
(803 Downloads)
| Abstract (HTML) (1685 Views)
Full-Text: (595 Views)
Introduction
Traumatic brain injury (TBI) is characterized by physical damage to brain tissue, resulting in either transient or enduring changes in brain function [1]. Neuropsychiatric outcomes experienced after TBI include a wide array of physical, cognitive, emotional, behavioural, and psychosocial difficulties [2]. Approximately 65% of individuals with moderate to severe TBI endure continuing cognitive deficits, particularly affecting memory, information processing, and executive function (EF) [3]. Executive dysfunction often emerges as a result of neurological injury to the frontal lobe, basal ganglia, thalamus, cerebellum, and the associated white matter pathways, which collectively constitute the fronto-subcortical circuits [4].
EF encompasses complex cognitive processes essential for planning, goal-oriented behaviour, social judgment, empathy and the anticipation of behavioural outcomes [5]. Deficits in these functions, especially in executive domains, can lead to significant impairments in occupational performance, social relationships, recreational activities, and daily living tasks. Such deficits also impose considerable economic burdens on patients, their families, and society [6]. These observations underscore the need for effective diagnostic tools to assess cognitive capacity and determine both the existence and seriousness of executive dysfunction.
Although the mini-mental state examination (MMSE) and the Montreal cognitive assessment (MoCA) evaluate cognitive impairments, both of them lack sufficient sensitivity to detect dysfunction specifically within the frontal lobes as screening tests [7, 8]. Traditional assessments, such as the MMSE, are more attuned to memory and language deficits and may miss early signs of executive dysfunction [9]. Moreover, research indicates that the MoCA can be time-intensive to administer and may be challenging for patients suffering from motor or speech deficits [8].
The frontal assessment battery (FAB) was developed as a functional tool to evaluate the existence and extent of executive dysfunction. It is easy to administer, requiring less than 10 minutes, and is well-received by patients [9]. Studies have shown that FAB scores correlate with various neuropsychological assessments of EF, including the Mattis dementia rating scale, Wisconsin card sorting test (WCST), and trail-making test [9, 10], as well as regional cerebral blood flow in the left callosomarginal and precentral regions, as measured by single-photon emission computed tomography [11].
The FAB has been proven to be a valid and reliable measure across various conditions, including, frontotemporal dementia [12], Huntington’s disease [13], Parkinson’s disease [14] and Alzheimer’s disease [15]. It has also been translated and validated in several languages, showing strong reliability metrics, such as Korean [16], Japanese [12], Chinese [17], German [18] and Italian [19]. In the Persian adaptation of the FAB, translated by Asaadi et al., it was applied to 49 individuals with Parkinson’s disease, achieving inter-rater reliability of 0.90 (confidence interval [CI]=0.77%, 0.95%). A linguistic adaptation was also made to the lexical fluency subtest by replacing the letter “S” with “B” to suit the phonetic characteristics of Farsi because “S” can lead to confusion among less-educated individuals due to phonetic variations [20].
While the FAB is clinically useful, its validity and reliability remain untested in patients with TBI (PwTBI), despite the frequent occurrence of executive dysfunction following moderate to severe TBI [6]. Consequently, our study was conducted to evaluate the reliability and validity of the FAB in individuals with TBI. Additionally, we sought to determine an optimal cut-off score for the FAB to effectively identify between PwTBI and healthy controls. We hypothesized that the FAB will be a valid and reliable tool for screening executive dysfunction in PwTBI.
Materials and Methods
This was a cross-sectional and descriptive-analytical study that received ethics approval from the University of Social Welfare and Rehabilitation Sciences ethics board. Sixty-five PwTBI (55 men, 10 women), aged 18 and 60, were recruited through University-affiliated rehabilitation centers in Tehran City, Iran. The inclusion criteria for TBI patients included a neurologist-confirmed TBI diagnosis, a cognitive functioning level of at least 6 on the Rancho Los Amigos scale, indicating goal-directed behaviour and consistent ability to follow simple directions [21], literacy and normal visual and auditory abilities. The exclusion criteria included patients with additional neurological disease (e.g. multiple sclerosis) or mental illness (e.g. schizophrenia). Table 1 presents the demographic and clinical profiles of the PwTBI.
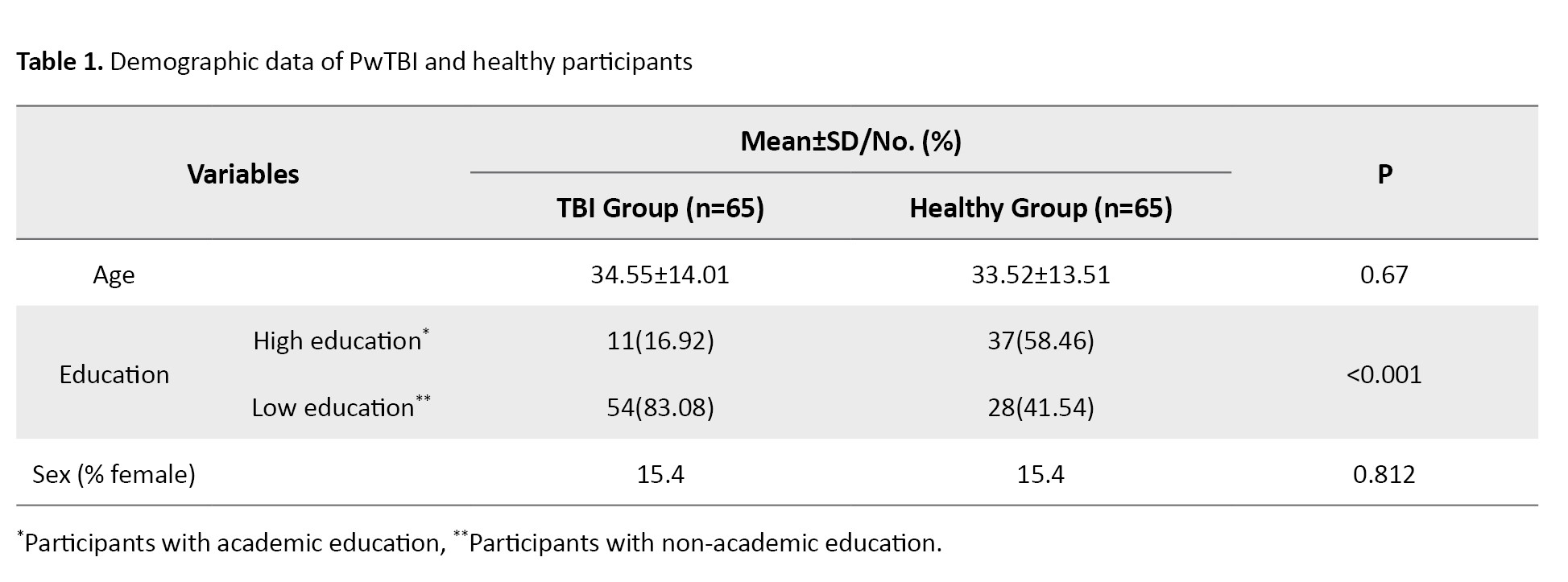
A comparison group of 65 healthy participants was matched to the TBI group by age and sex. The inclusion criteria for healthy controls included the absence of any history of neurological or psychiatric disorders, hearing impairments, or visual disabilities. Participants were selected via convenience sampling, with stratified sampling overseen by the study investigator. Exclusion from the study occurred if participants withdrew consent or experienced seizures during testing. Exclusion decisions were made in consultation with a neurologist.
Measures of EF
The Persian frontal assessment battery (P-FAB) included six subtests, conceptualization (recognizing similarities between objects), lexical fluency (number of words beginning with “B” provided in 60 seconds), motor programming (fist-palm-edge motor sequence), sensitivity to interference (performing tasks that require responding in opposition to the given signal), inhibitory control (go/no-go paradigm) and environmental autonomy (inhibition of grasping behaviour). Scores for each subtest range from 0 to 3, with lower scores reflecting more severe executive dysfunction and a total maximum score of 18 [9]. The P-FAB has demonstrated high intra-judge reliability in individuals with Parkinson’s disease (r=0.9) [20].
The WCST is a widely employed neuropsychological assessment for EF, measuring abilities in concept formation, planning, cognitive flexibility, visuospatial working memory, deductive reasoning, problem-solving, and set-shifting [22]. The Heaton’s 64-card version of the WCST was used in this study [23, 24]. Among the Iranian population, the WCST has shown strong test re-test reliability, with a coefficient of 0.85 [25].
The Stroop colour and word test (SCWT) is a well-established neuropsychological tool used to evaluate processing speed (colour and word naming), cognitive flexibility (switching conditions) and inhibition of cognitive interference, known as the Stroop effect, where processing one feature of a stimulus interferes with simultaneous processing of another [26]. Among the Iranian population, this test demonstrated a test re-test reliability coefficient of 0.71 [27].
Procedures
An experienced occupational therapist conducted interviews with all participants (both patients and healthy controls), explaining the study’s purpose and procedures. Eligible participants provided written informed consent. Each participant was assessed using demographic data collection, the MMSE, WCST, Stroop test and the P-FAB. The study comprised three phases, reliability testing (including test re-test and inter-judge reliability), validity assessment (concurrent and discriminant), and identifying an optimal threshold score for the total FAB to differentiate PwTBI from healthy controls.
For inter-judge reliability, two raters independently scored 20 patients simultaneously. Test re-test reliability was estimated in a subsample of 30 patients who retook the P- FAB two weeks later [17, 19]. Test re-test and inter-judge reliabilities were assessed using the intraclass correlation coefficient (ICC), calculated from a two-way random effects model with absolute agreement and average measure. Reliability was classified as fair (0.40–0.59), good (0.60–0.75), or excellent (≥0.75) [12]. Additionally, standard error of measurement (SEM) and minimal detectable change (MDC) were calculated using the Equations 1 and 2:
1. SD_√1-ICC
2. 1.96×SEM×√2, respectively.
Concurrent validity was assessed by calculating Spearman correlation coefficients between the P-FAB and scores on the WCST, MMSE, and Stroop test, with the tests administered in a random order to the patients. Discriminant validity was assessed by comparing the average FAB scores of TBI patients with healthy controls [28]. A receiver operating characteristic curve analysis determined the cut-off for the FAB total score to identify TBI patients from controls, with sensitivity and specificity calculated to validate this threshold.
Statistical Analysis
Descriptive statistics, including frequencies and means, were used to summarize participant demographics. Mean was compared using t tests or the Mann-Whitney U test, depending on data distribution. Spearman correlation tests assessed relationships between FAB scores and variables, such as sex, age, and education. Reliability (test re-test and inter-judge) was measured using ICC analysis with a 95% CI. Post-hoc analysis in analysis of variance (ANOVA) was used for multiple comparisons. The Youden index identified the optimal FAB cut-off score, with the highest index indicating the best threshold [29]. Analyses were conducted using SPSS software, version 22, with statistical significance set at P<0.05.
Results
Demographic data
A total of 130 participants (65 PwTBI and 65 healthy controls) completed the study. Table 1 presents the demographic details of both groups. No significant relationships were identified between the P-FAB total score and demographic variables among PwTBI (P>0.05). However, a significant relationship was found between the P-FAB overall score and age in the healthy control group (P<0.05) (Table 2).
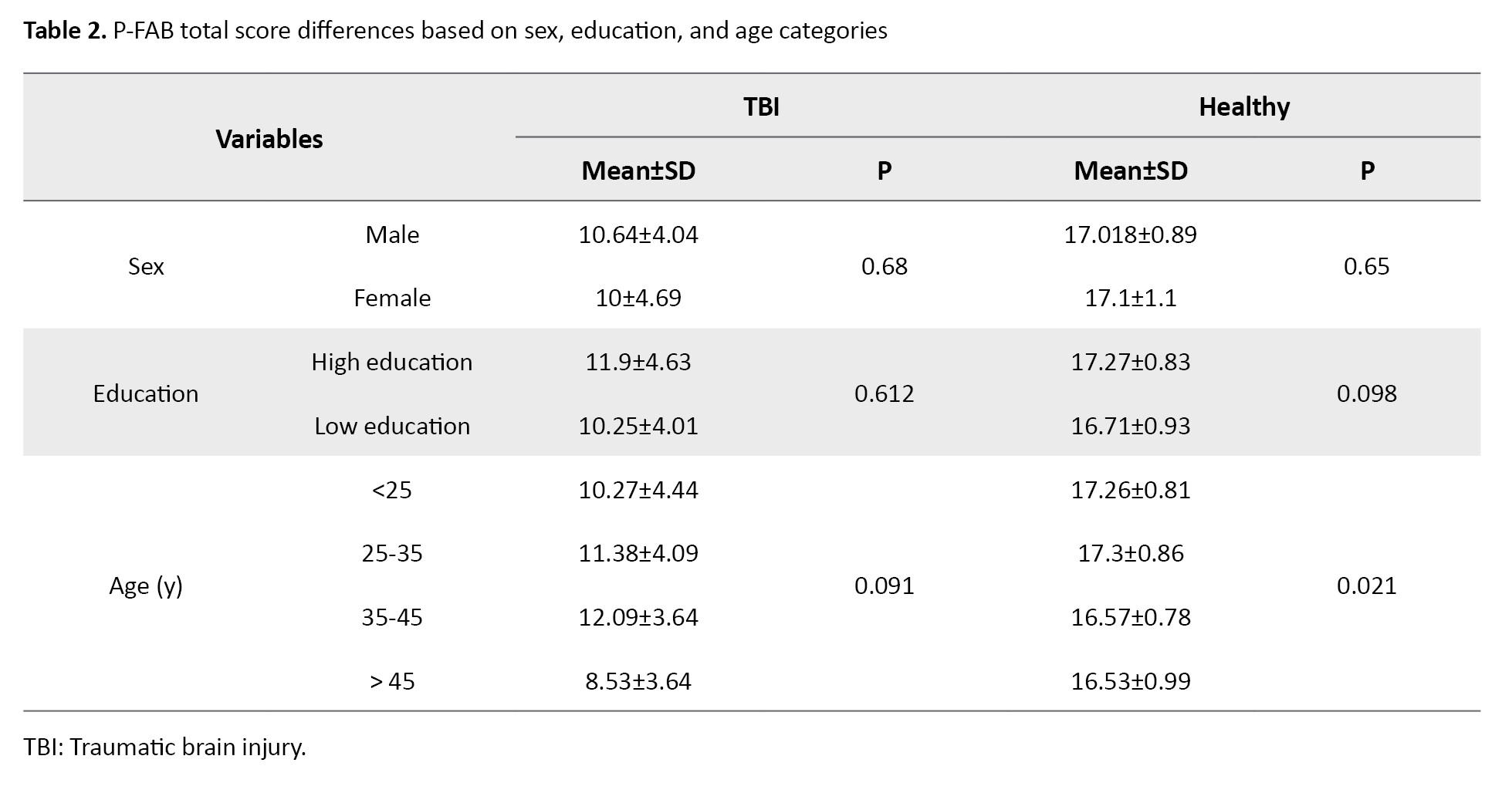
Post-hoc analysis using analysis of variance (ANOVA) indicated no significant differences in P-FAB total scores across specific age groups within the healthy control group at a conventional significance level (P>0.05). However, at a 0.10 significance level, significant differences were observed between participants under 25 years and those over 45, as well as between participants aged 25–35 and those over 45.
Reliability
Table 3 presents the test re-test and inter-judge reliability results for the P-FAB.
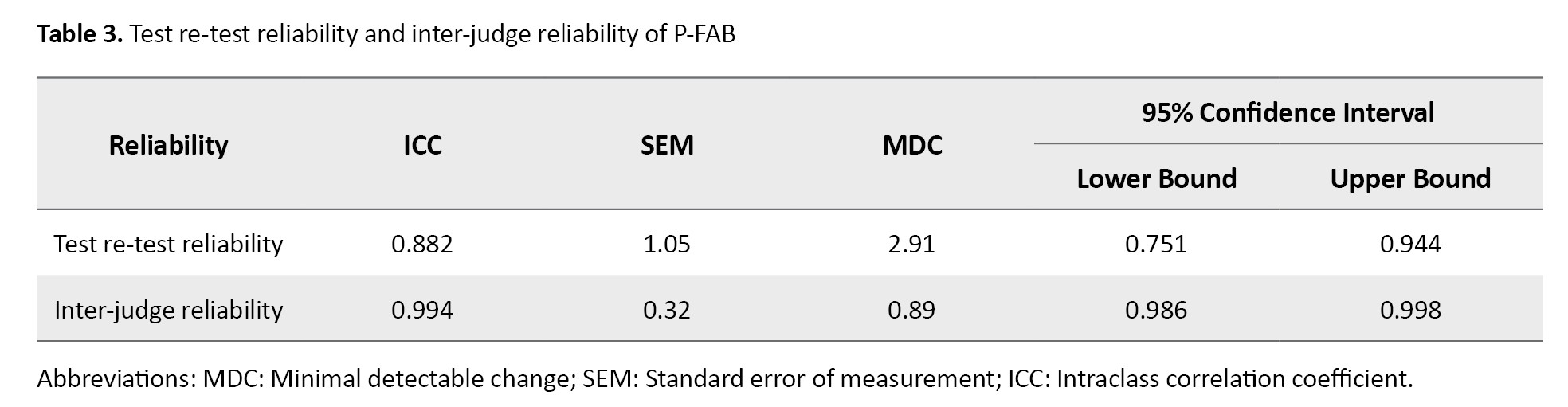
This version exhibited strong internal consistency across all six subtests, with a Cronbach’s α coefficient of 0.863. Test re-test reliability (n=30) assessed after a two-week interval showed excellent stability, with an ICC of 0.882 (95% CI, 0.77%, 0.95%). Inter-judge reliability was also outstanding, with an ICC of 0.994 (95% CI, 0.77%, 0.95%), further confirming excellent reliability in both test re-test and inter-judge measures for the P-FAB. Table 3 presents the SEM and MDC.
Validity
Concurrent Validity
Table 4 presents the results for the concurrent validity of the P-FAB about the MMSE, WCST, and Stroop tests.
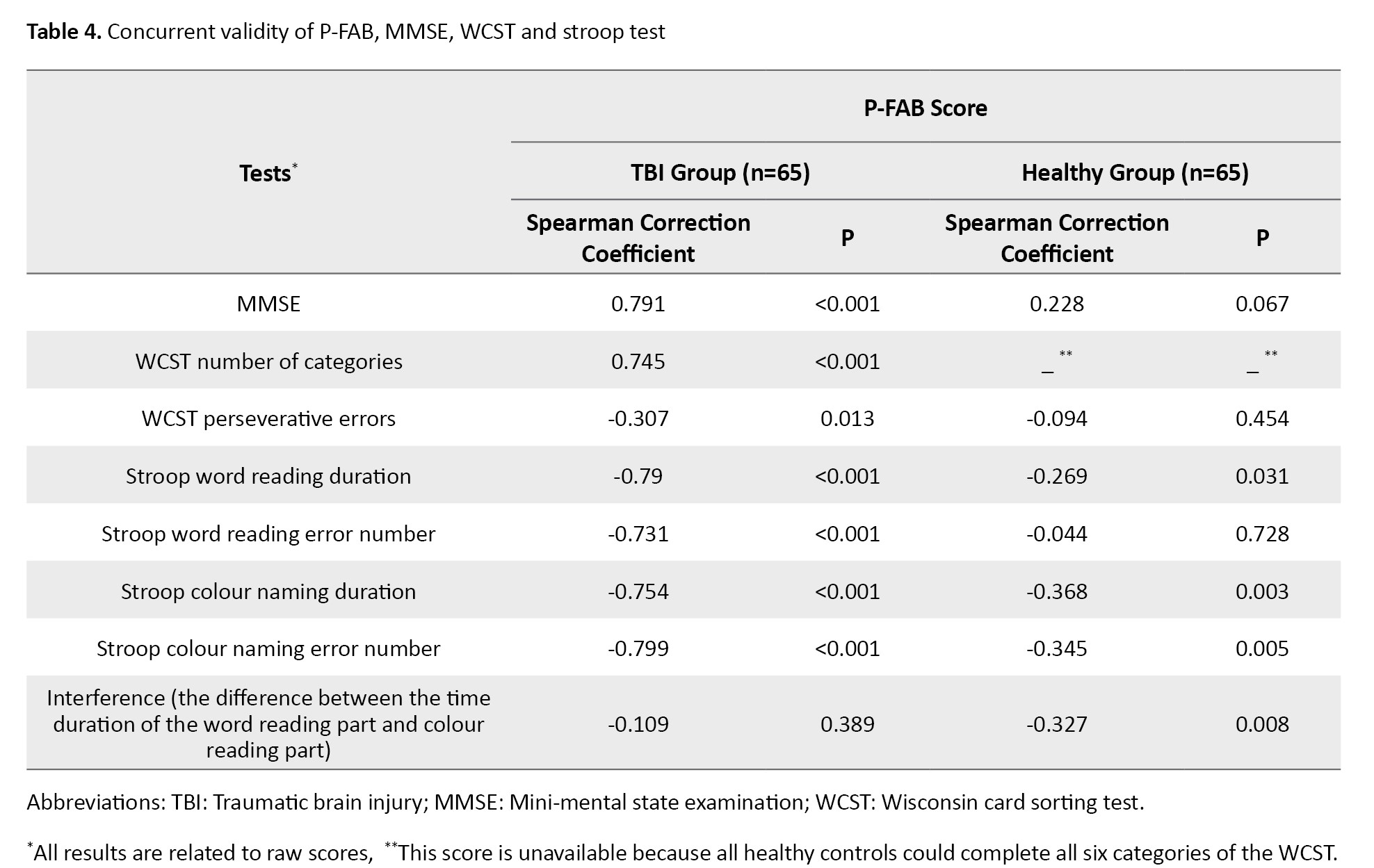
A strong and statistically significant correlation was observed between the total P-FAB score and the MMSE score (r=0.791, P<0.001). Furthermore, the P-FAB score showed a significant correlation with the number of categories completed on the WCST (r=0.745, P<0.001) and the number of perseverative errors (r=0.307, P<0.05). Additionally, significant correlations were found between the P-FAB score and all conditions of the Stroop test (P<0.001), except for the interference score, which assesses the difference between word reading and colour naming times, where no significant correlation was observed (P>0.05).
Discriminant validity
Table 5 presents the subtest scores of the P-FAB for both PwTBI and healthy control groups.
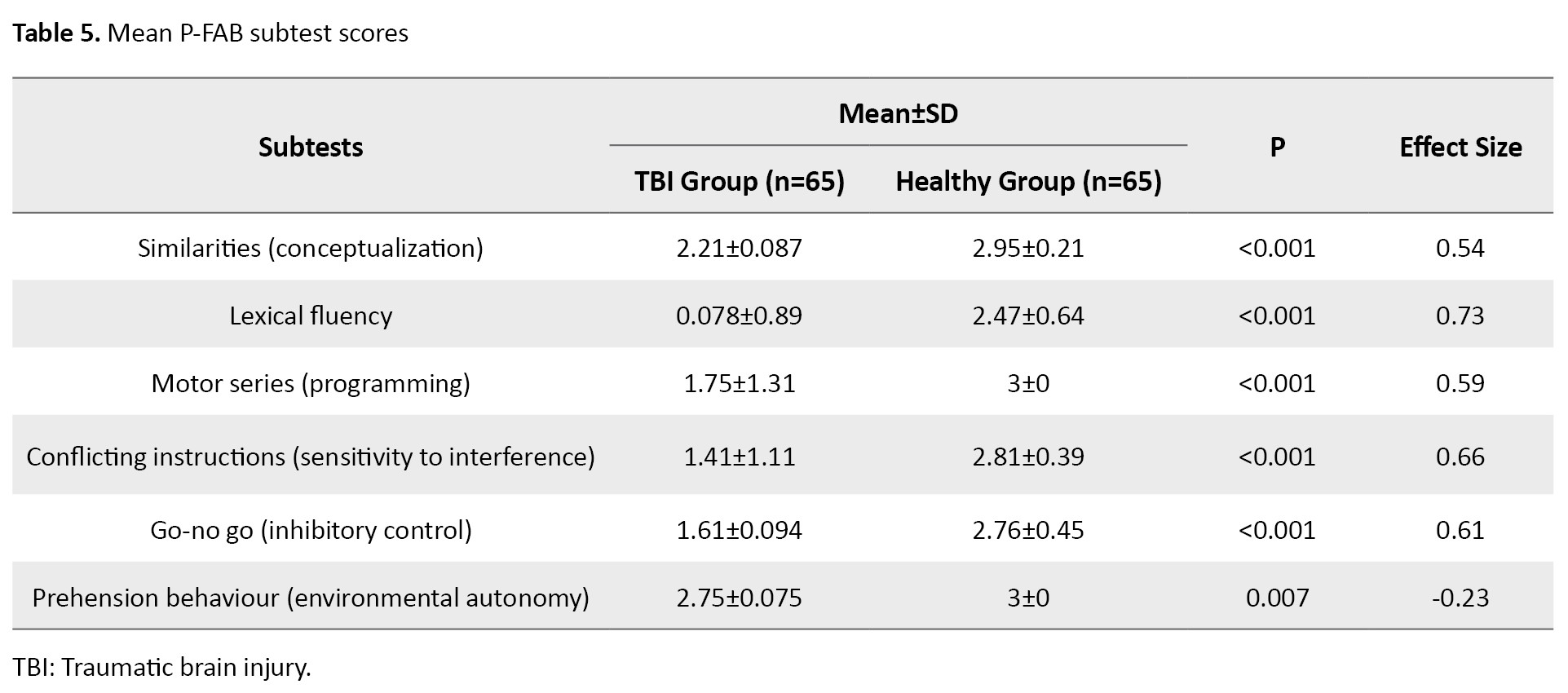
Analysis using the Mann-Whitney test revealed a significant group effect on the total P-FAB score and each subtest score (P<0.01). The PwTBI scored lower both on the total P-FAB and across each subtest compared to healthy controls, supporting the P-FAB’s strong discriminant validity in distinguishing between these groups.
Table 6 shows that a P-FAB score cut-off of 15 provided excellent sensitivity and specificity for distinguishing PwTBI from healthy participants.
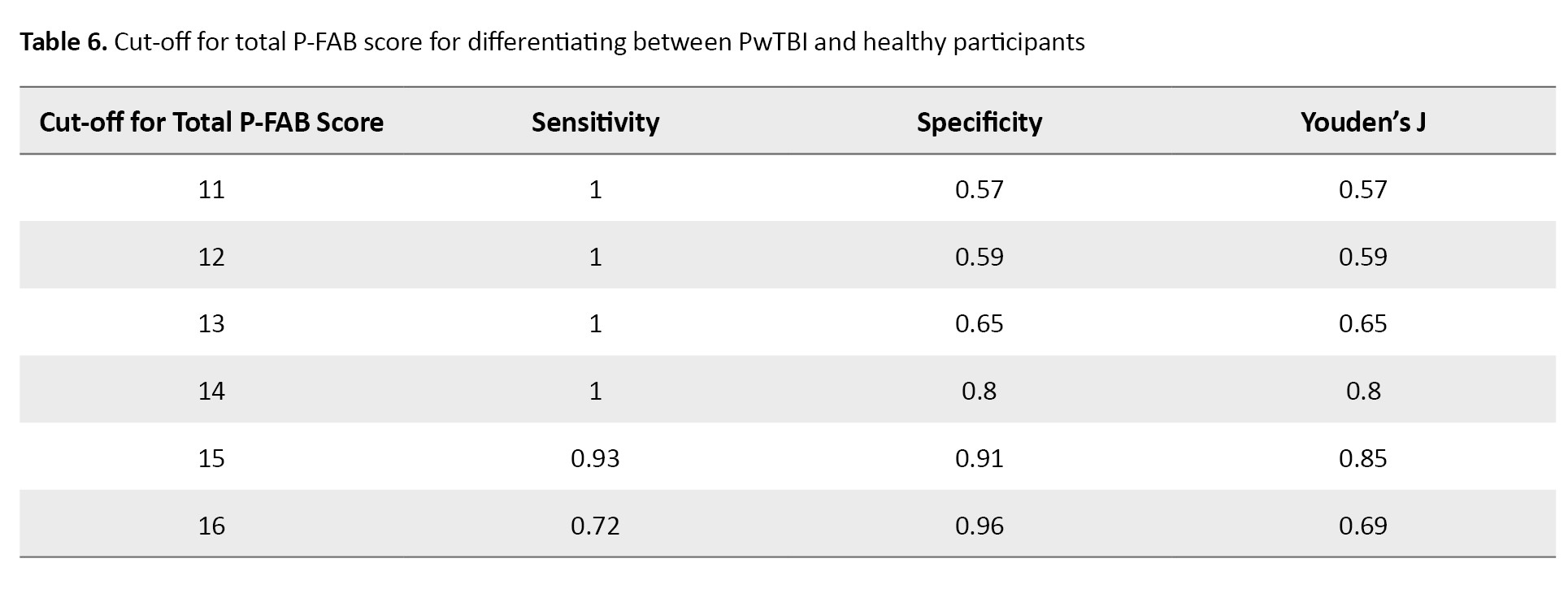
This cut-off yielded a sensitivity of 0.98 and a specificity of 0.90, indicating high accuracy in identifying TBI-related executive dysfunction.
Discussion
This research was conducted to evaluate the validity and reliability of the (P-FAB) in PwTBI. Additionally, we sought to establish a suitable cut-off score for the P-FAB to distinguish between PwTBI and healthy controls.
Previous studies have indicated that age, sex, and education do not influence FAB scores in patients with dementia [30, 31], a finding corroborated by our study. However, other research has shown a positive correlation between education and FAB scores and a negative correlation with age among healthy participants and those with Parkinson’s disease [14, 20]. This discrepancy may be due to the younger average age and broader age range of participants in our study. Furthermore, unlike Parkinson’s disease, which is degenerative and leads to progressive cognitive decline, TBI is not a degenerative condition [32]. A considerable proportion of present PwTBI also had less education attainment. This is consistent with reports showing a rising prevalence of TBI in economically disadvantaged and middle-income countries due to increased motor vehicle usage and the greater likelihood of people with lower education being employed in high-risk jobs [33].
Our results showed that the FAB demonstrates strong test re-test and inter-judge reliability, in addition to acceptable concurrent and discriminant validity. Moreover, present results revealed significant correlations between the P-FAB and other tests evaluating frontal lobe functions, including the MMSE, WCST, and various conditions of the Stroop test, confirming the FAB’s concurrent validity for measuring executive dysfunction [9, 17, 20, 34, 35]. The FAB showed a strong correlation with the MMSE. However, since the MMSE assesses non-EFs, this brings into question the FAB’s ability to discriminate in measuring frontal lobe functions specific to TBI. A more detailed subgroup analysis would be beneficial for further understanding the relationship between FAB and cognitive assessments, such as the MMSE. Moreover, the FAB was significantly correlated with WCST performance, particularly in terms of the number of categories achieved and perseveration errors. Since perseveration errors in WCST are associated with executive dysfunction, this suggests that the FAB is effective in evaluating executive dysfunction.
The present study also revealed significant relationships between the Stroop test’s time length and error rates, even though these measures primarily assess processing speed and cognitive flexibility rather than EFs. Additionally, the absence of a significant correlation between the FAB and the Stroop interference score, which directly measures EFs, could indicate that different regions within the frontal lobe underlie the tasks assessed by each test [11, 36, 37]. Stroop performance, for instance, is thought to involve the left frontal region, particularly the anterior cingulate cortex and orbital parts of the prefrontal cortex, while other studies suggest that FAB functions may be more associated with the left precentral and bilateral callosomarginal areas [11, 38].
Literature showed significant differences in FAB scores between patients with small sub-cortical infarcts and also patients with Alzheimer’s disease compared to the control group [16, 17]. Consistent with these results, our study found that the FAB can effectively distinguish between healthy individuals and TBI patients. Given its stronger correlation with the MMSE compared to WCST, the FAB may be especially useful for identifying mild cognitive impairments that other tests may overlook (WSCT, Stroop test) and for assessing the severity of executive dysfunction, with lower scores indicating more pronounced dysfunction [9]. Thus, the FAB is a promising functional screening tool in clinical situations.
In this study, we determined a cut-off score of 15 for the FAB as optimal for differentiating TBI patients from healthy individuals, providing high sensitivity (0.93) and specificity (0.9). Adjusting the cut-off below 15 reduced specificity, while increasing it beyond 15 lowered sensitivity, as detailed in Table 6.
Conclusion
This research demonstrated that the P-FAB provides adequate validity and reliability to evaluate frontal lobe functions and executive dysfunction in PwTBI, effectively distinguishing them from healthy participants. We recommend the P-FAB as a rapid, convenient assessment tool for TBI screening. Further research using computer-based tests is recommended to validate these findings.
Limitations
This study had limitations regarding patient accessibility for retesting and challenges in encouraging participation. The hospital permission process for sampling also restricted our efforts. Additionally, we lacked information on injury severity and time since injury for some patients, as well as subgroup scores for the MMSE, which limited our methodology.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics committee of the University of Social Welfare and Rehabilitation Sciences (Code: IR.USWR.REC.1397.016).
Funding
The paper was extracted from the master thesis of Ramin Banimahdi, approved by University of Ottawa.
Authors' contributions
Conceptualization and supervision: Nazila Akbarfahimi, Seyed Ali Hosseini and Seyed Majid Akhavan Hejazi; Methodology: Mohsen Vahedi and Ramin Banimahdi; Writing the original draft: Ramin Banimahdi; Investigation, review & editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors express their gratitude to Rofeideh Rehabilitation Hospital Clinical Research Development Center, the University of Social Welfare and Rehabilitation Sciences, the Clinical Research Development Units of Loghman Hakim, Shohada-e-Tajrish Hospital, Firoozgar Hospitals, Shahid Beheshti University of Medical Sciences, Rasoul Akram Hospital and Iran University of Medical Sciences, Tehran, Iran, for their support, cooperation and assistance throughout this study.
References
Traumatic brain injury (TBI) is characterized by physical damage to brain tissue, resulting in either transient or enduring changes in brain function [1]. Neuropsychiatric outcomes experienced after TBI include a wide array of physical, cognitive, emotional, behavioural, and psychosocial difficulties [2]. Approximately 65% of individuals with moderate to severe TBI endure continuing cognitive deficits, particularly affecting memory, information processing, and executive function (EF) [3]. Executive dysfunction often emerges as a result of neurological injury to the frontal lobe, basal ganglia, thalamus, cerebellum, and the associated white matter pathways, which collectively constitute the fronto-subcortical circuits [4].
EF encompasses complex cognitive processes essential for planning, goal-oriented behaviour, social judgment, empathy and the anticipation of behavioural outcomes [5]. Deficits in these functions, especially in executive domains, can lead to significant impairments in occupational performance, social relationships, recreational activities, and daily living tasks. Such deficits also impose considerable economic burdens on patients, their families, and society [6]. These observations underscore the need for effective diagnostic tools to assess cognitive capacity and determine both the existence and seriousness of executive dysfunction.
Although the mini-mental state examination (MMSE) and the Montreal cognitive assessment (MoCA) evaluate cognitive impairments, both of them lack sufficient sensitivity to detect dysfunction specifically within the frontal lobes as screening tests [7, 8]. Traditional assessments, such as the MMSE, are more attuned to memory and language deficits and may miss early signs of executive dysfunction [9]. Moreover, research indicates that the MoCA can be time-intensive to administer and may be challenging for patients suffering from motor or speech deficits [8].
The frontal assessment battery (FAB) was developed as a functional tool to evaluate the existence and extent of executive dysfunction. It is easy to administer, requiring less than 10 minutes, and is well-received by patients [9]. Studies have shown that FAB scores correlate with various neuropsychological assessments of EF, including the Mattis dementia rating scale, Wisconsin card sorting test (WCST), and trail-making test [9, 10], as well as regional cerebral blood flow in the left callosomarginal and precentral regions, as measured by single-photon emission computed tomography [11].
The FAB has been proven to be a valid and reliable measure across various conditions, including, frontotemporal dementia [12], Huntington’s disease [13], Parkinson’s disease [14] and Alzheimer’s disease [15]. It has also been translated and validated in several languages, showing strong reliability metrics, such as Korean [16], Japanese [12], Chinese [17], German [18] and Italian [19]. In the Persian adaptation of the FAB, translated by Asaadi et al., it was applied to 49 individuals with Parkinson’s disease, achieving inter-rater reliability of 0.90 (confidence interval [CI]=0.77%, 0.95%). A linguistic adaptation was also made to the lexical fluency subtest by replacing the letter “S” with “B” to suit the phonetic characteristics of Farsi because “S” can lead to confusion among less-educated individuals due to phonetic variations [20].
While the FAB is clinically useful, its validity and reliability remain untested in patients with TBI (PwTBI), despite the frequent occurrence of executive dysfunction following moderate to severe TBI [6]. Consequently, our study was conducted to evaluate the reliability and validity of the FAB in individuals with TBI. Additionally, we sought to determine an optimal cut-off score for the FAB to effectively identify between PwTBI and healthy controls. We hypothesized that the FAB will be a valid and reliable tool for screening executive dysfunction in PwTBI.
Materials and Methods
This was a cross-sectional and descriptive-analytical study that received ethics approval from the University of Social Welfare and Rehabilitation Sciences ethics board. Sixty-five PwTBI (55 men, 10 women), aged 18 and 60, were recruited through University-affiliated rehabilitation centers in Tehran City, Iran. The inclusion criteria for TBI patients included a neurologist-confirmed TBI diagnosis, a cognitive functioning level of at least 6 on the Rancho Los Amigos scale, indicating goal-directed behaviour and consistent ability to follow simple directions [21], literacy and normal visual and auditory abilities. The exclusion criteria included patients with additional neurological disease (e.g. multiple sclerosis) or mental illness (e.g. schizophrenia). Table 1 presents the demographic and clinical profiles of the PwTBI.

A comparison group of 65 healthy participants was matched to the TBI group by age and sex. The inclusion criteria for healthy controls included the absence of any history of neurological or psychiatric disorders, hearing impairments, or visual disabilities. Participants were selected via convenience sampling, with stratified sampling overseen by the study investigator. Exclusion from the study occurred if participants withdrew consent or experienced seizures during testing. Exclusion decisions were made in consultation with a neurologist.
Measures of EF
The Persian frontal assessment battery (P-FAB) included six subtests, conceptualization (recognizing similarities between objects), lexical fluency (number of words beginning with “B” provided in 60 seconds), motor programming (fist-palm-edge motor sequence), sensitivity to interference (performing tasks that require responding in opposition to the given signal), inhibitory control (go/no-go paradigm) and environmental autonomy (inhibition of grasping behaviour). Scores for each subtest range from 0 to 3, with lower scores reflecting more severe executive dysfunction and a total maximum score of 18 [9]. The P-FAB has demonstrated high intra-judge reliability in individuals with Parkinson’s disease (r=0.9) [20].
The WCST is a widely employed neuropsychological assessment for EF, measuring abilities in concept formation, planning, cognitive flexibility, visuospatial working memory, deductive reasoning, problem-solving, and set-shifting [22]. The Heaton’s 64-card version of the WCST was used in this study [23, 24]. Among the Iranian population, the WCST has shown strong test re-test reliability, with a coefficient of 0.85 [25].
The Stroop colour and word test (SCWT) is a well-established neuropsychological tool used to evaluate processing speed (colour and word naming), cognitive flexibility (switching conditions) and inhibition of cognitive interference, known as the Stroop effect, where processing one feature of a stimulus interferes with simultaneous processing of another [26]. Among the Iranian population, this test demonstrated a test re-test reliability coefficient of 0.71 [27].
Procedures
An experienced occupational therapist conducted interviews with all participants (both patients and healthy controls), explaining the study’s purpose and procedures. Eligible participants provided written informed consent. Each participant was assessed using demographic data collection, the MMSE, WCST, Stroop test and the P-FAB. The study comprised three phases, reliability testing (including test re-test and inter-judge reliability), validity assessment (concurrent and discriminant), and identifying an optimal threshold score for the total FAB to differentiate PwTBI from healthy controls.
For inter-judge reliability, two raters independently scored 20 patients simultaneously. Test re-test reliability was estimated in a subsample of 30 patients who retook the P- FAB two weeks later [17, 19]. Test re-test and inter-judge reliabilities were assessed using the intraclass correlation coefficient (ICC), calculated from a two-way random effects model with absolute agreement and average measure. Reliability was classified as fair (0.40–0.59), good (0.60–0.75), or excellent (≥0.75) [12]. Additionally, standard error of measurement (SEM) and minimal detectable change (MDC) were calculated using the Equations 1 and 2:
1. SD_√1-ICC
2. 1.96×SEM×√2, respectively.
Concurrent validity was assessed by calculating Spearman correlation coefficients between the P-FAB and scores on the WCST, MMSE, and Stroop test, with the tests administered in a random order to the patients. Discriminant validity was assessed by comparing the average FAB scores of TBI patients with healthy controls [28]. A receiver operating characteristic curve analysis determined the cut-off for the FAB total score to identify TBI patients from controls, with sensitivity and specificity calculated to validate this threshold.
Statistical Analysis
Descriptive statistics, including frequencies and means, were used to summarize participant demographics. Mean was compared using t tests or the Mann-Whitney U test, depending on data distribution. Spearman correlation tests assessed relationships between FAB scores and variables, such as sex, age, and education. Reliability (test re-test and inter-judge) was measured using ICC analysis with a 95% CI. Post-hoc analysis in analysis of variance (ANOVA) was used for multiple comparisons. The Youden index identified the optimal FAB cut-off score, with the highest index indicating the best threshold [29]. Analyses were conducted using SPSS software, version 22, with statistical significance set at P<0.05.
Results
Demographic data
A total of 130 participants (65 PwTBI and 65 healthy controls) completed the study. Table 1 presents the demographic details of both groups. No significant relationships were identified between the P-FAB total score and demographic variables among PwTBI (P>0.05). However, a significant relationship was found between the P-FAB overall score and age in the healthy control group (P<0.05) (Table 2).

Post-hoc analysis using analysis of variance (ANOVA) indicated no significant differences in P-FAB total scores across specific age groups within the healthy control group at a conventional significance level (P>0.05). However, at a 0.10 significance level, significant differences were observed between participants under 25 years and those over 45, as well as between participants aged 25–35 and those over 45.
Reliability
Table 3 presents the test re-test and inter-judge reliability results for the P-FAB.

This version exhibited strong internal consistency across all six subtests, with a Cronbach’s α coefficient of 0.863. Test re-test reliability (n=30) assessed after a two-week interval showed excellent stability, with an ICC of 0.882 (95% CI, 0.77%, 0.95%). Inter-judge reliability was also outstanding, with an ICC of 0.994 (95% CI, 0.77%, 0.95%), further confirming excellent reliability in both test re-test and inter-judge measures for the P-FAB. Table 3 presents the SEM and MDC.
Validity
Concurrent Validity
Table 4 presents the results for the concurrent validity of the P-FAB about the MMSE, WCST, and Stroop tests.

A strong and statistically significant correlation was observed between the total P-FAB score and the MMSE score (r=0.791, P<0.001). Furthermore, the P-FAB score showed a significant correlation with the number of categories completed on the WCST (r=0.745, P<0.001) and the number of perseverative errors (r=0.307, P<0.05). Additionally, significant correlations were found between the P-FAB score and all conditions of the Stroop test (P<0.001), except for the interference score, which assesses the difference between word reading and colour naming times, where no significant correlation was observed (P>0.05).
Discriminant validity
Table 5 presents the subtest scores of the P-FAB for both PwTBI and healthy control groups.

Analysis using the Mann-Whitney test revealed a significant group effect on the total P-FAB score and each subtest score (P<0.01). The PwTBI scored lower both on the total P-FAB and across each subtest compared to healthy controls, supporting the P-FAB’s strong discriminant validity in distinguishing between these groups.
Table 6 shows that a P-FAB score cut-off of 15 provided excellent sensitivity and specificity for distinguishing PwTBI from healthy participants.

This cut-off yielded a sensitivity of 0.98 and a specificity of 0.90, indicating high accuracy in identifying TBI-related executive dysfunction.
Discussion
This research was conducted to evaluate the validity and reliability of the (P-FAB) in PwTBI. Additionally, we sought to establish a suitable cut-off score for the P-FAB to distinguish between PwTBI and healthy controls.
Previous studies have indicated that age, sex, and education do not influence FAB scores in patients with dementia [30, 31], a finding corroborated by our study. However, other research has shown a positive correlation between education and FAB scores and a negative correlation with age among healthy participants and those with Parkinson’s disease [14, 20]. This discrepancy may be due to the younger average age and broader age range of participants in our study. Furthermore, unlike Parkinson’s disease, which is degenerative and leads to progressive cognitive decline, TBI is not a degenerative condition [32]. A considerable proportion of present PwTBI also had less education attainment. This is consistent with reports showing a rising prevalence of TBI in economically disadvantaged and middle-income countries due to increased motor vehicle usage and the greater likelihood of people with lower education being employed in high-risk jobs [33].
Our results showed that the FAB demonstrates strong test re-test and inter-judge reliability, in addition to acceptable concurrent and discriminant validity. Moreover, present results revealed significant correlations between the P-FAB and other tests evaluating frontal lobe functions, including the MMSE, WCST, and various conditions of the Stroop test, confirming the FAB’s concurrent validity for measuring executive dysfunction [9, 17, 20, 34, 35]. The FAB showed a strong correlation with the MMSE. However, since the MMSE assesses non-EFs, this brings into question the FAB’s ability to discriminate in measuring frontal lobe functions specific to TBI. A more detailed subgroup analysis would be beneficial for further understanding the relationship between FAB and cognitive assessments, such as the MMSE. Moreover, the FAB was significantly correlated with WCST performance, particularly in terms of the number of categories achieved and perseveration errors. Since perseveration errors in WCST are associated with executive dysfunction, this suggests that the FAB is effective in evaluating executive dysfunction.
The present study also revealed significant relationships between the Stroop test’s time length and error rates, even though these measures primarily assess processing speed and cognitive flexibility rather than EFs. Additionally, the absence of a significant correlation between the FAB and the Stroop interference score, which directly measures EFs, could indicate that different regions within the frontal lobe underlie the tasks assessed by each test [11, 36, 37]. Stroop performance, for instance, is thought to involve the left frontal region, particularly the anterior cingulate cortex and orbital parts of the prefrontal cortex, while other studies suggest that FAB functions may be more associated with the left precentral and bilateral callosomarginal areas [11, 38].
Literature showed significant differences in FAB scores between patients with small sub-cortical infarcts and also patients with Alzheimer’s disease compared to the control group [16, 17]. Consistent with these results, our study found that the FAB can effectively distinguish between healthy individuals and TBI patients. Given its stronger correlation with the MMSE compared to WCST, the FAB may be especially useful for identifying mild cognitive impairments that other tests may overlook (WSCT, Stroop test) and for assessing the severity of executive dysfunction, with lower scores indicating more pronounced dysfunction [9]. Thus, the FAB is a promising functional screening tool in clinical situations.
In this study, we determined a cut-off score of 15 for the FAB as optimal for differentiating TBI patients from healthy individuals, providing high sensitivity (0.93) and specificity (0.9). Adjusting the cut-off below 15 reduced specificity, while increasing it beyond 15 lowered sensitivity, as detailed in Table 6.
Conclusion
This research demonstrated that the P-FAB provides adequate validity and reliability to evaluate frontal lobe functions and executive dysfunction in PwTBI, effectively distinguishing them from healthy participants. We recommend the P-FAB as a rapid, convenient assessment tool for TBI screening. Further research using computer-based tests is recommended to validate these findings.
Limitations
This study had limitations regarding patient accessibility for retesting and challenges in encouraging participation. The hospital permission process for sampling also restricted our efforts. Additionally, we lacked information on injury severity and time since injury for some patients, as well as subgroup scores for the MMSE, which limited our methodology.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics committee of the University of Social Welfare and Rehabilitation Sciences (Code: IR.USWR.REC.1397.016).
Funding
The paper was extracted from the master thesis of Ramin Banimahdi, approved by University of Ottawa.
Authors' contributions
Conceptualization and supervision: Nazila Akbarfahimi, Seyed Ali Hosseini and Seyed Majid Akhavan Hejazi; Methodology: Mohsen Vahedi and Ramin Banimahdi; Writing the original draft: Ramin Banimahdi; Investigation, review & editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors express their gratitude to Rofeideh Rehabilitation Hospital Clinical Research Development Center, the University of Social Welfare and Rehabilitation Sciences, the Clinical Research Development Units of Loghman Hakim, Shohada-e-Tajrish Hospital, Firoozgar Hospitals, Shahid Beheshti University of Medical Sciences, Rasoul Akram Hospital and Iran University of Medical Sciences, Tehran, Iran, for their support, cooperation and assistance throughout this study.
References
- Pervez M, Kitagawa RS, Chang TR. Definition of traumatic brain injury, neurosurgery, trauma orthopedics, neuroimaging, psychology, and psychiatry in mild traumatic brain injury. Neuroimaging Clin N Am. 2018; 28(1):1-13. [DOI:10.1016/j.nic.2017.09.010] [PMID]
- Silver JM, McAllister TW, Arciniegas DB. Depression and cognitive complaints following mild traumatic brain injury. Am J Psychiatry. 2009; 166(6):653-61. [DOI:10.1176/appi.ajp.2009.08111676] [PMID]
- Peng Q, Yang J, Strome T, Weldon E, Chochinov A. Bottleneck detection and reduction using simulation modeling to Reduce Overcrowding of Hospital Emergency Department. J Model Optim. 2020; 12(2):100-9. [DOI:10.32732/jmo.2020.12.2.100]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986; 9:357-81. [DOI:10.1146/annurev.ne.09.030186.002041] [PMID]
- Moorhouse P, Gorman M, Rockwood K. Comparison of EXIT-25 and the Frontal Assessment Battery for evaluation of executive dysfunction in patients attending a memory clinic. Dement Geriatr Cogn Disord. 2009; 27(5):424-8. [DOI:10.1159/000212755] [PMID]
- Rabinowitz AR, Levin HS. Cognitive sequelae of traumatic brain injury. Psychiatr Clin North Am. 2014; 37(1):1-11. [DOI:10.1016/j.psc.2013.11.004] [PMID]
- Marconi R, Antonini A, Barone P, Colosimo C, Avarello TP, Bottacchi E, et al. Frontal assessment battery scores and non-motor symptoms in parkinsonian disorders. Neurol Sci. 2012; 33(3):585-93. [DOI:10.1007/s10072-011-0807-x] [PMID]
- Osborne RA, Sekhon R, Johnston W, Kalra S. Screening for frontal lobe and general cognitive impairment in patients with amyotrophic lateral sclerosis. J Neurol Sci. 2014; 336(1-2):191-6. [DOI:10.1016/j.jns.2013.10.038] [PMID]
- Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: A frontal assessment battery at bedside. Neurology. 2000; 55(11):1621-6. [DOI:10.1212/WNL.55.11.1621] [PMID]
- Cohen OS, Vakil E, Tanne D, Molshatzki N, Nitsan Z, Hassin-Baer S. The frontal assessment battery as a tool for evaluation of frontal lobe dysfunction in patients with Parkinson disease. J Geriatr Psychiatry Neurol. 2012; 25(2):71-7. [DOI:10.1177/0891988712445087] [PMID]
- Kume K, Hanyu H, Murakami M, Sato T, Hirao K, Kanetaka H, et al. Frontal Assessment Battery and brain perfusion images in amnestic mild cognitive impairment. Geriatr Gerontol Int. 2011; 11(1):77-82. [DOI:10.1111/j.1447-0594.2010.00645.x] [PMID]
- Nakaaki S, Murata Y, Sato J, Shinagawa Y, Matsui T, Tatsumi H, et al. Reliability and validity of the Japanese version of the Frontal Assessment Battery in patients with the frontal variant of frontotemporal dementia. Psychiatry Clin Neurosci. 2007; 61(1):78-83.[DOI:10.1111/j.1440-1819.2007.01614.x] [PMID]
- Rodrigues GR, Souza CP, Cetlin RS, de Oliveira DS, Pena-Pereira M, Ujikawa LT, et al. Use of the frontal assessment battery in evaluating executive dysfunction in patients with Huntington’s disease. J Neurol. 2009; 256(11):1809-15. [DOI:10.1007/s00415-009-5197-0] [PMID]
- Lima CF, Meireles LP, Fonseca R, Castro SL, Garrett C. The Frontal Assessment Battery (FAB) in Parkinson’s disease and correlations with formal measures of executive functioning. J Neurol. 2008; 255(11):1756-61. [DOI:10.1007/s00415-008-0024-6] [PMID]
- Nagata T, Shinagawa S, Ochiai Y, Kada H, Kasahara H, Nukariya K, et al. Relationship of frontal lobe dysfunction and aberrant motor behaviors in patients with Alzheimer’s disease. Int Psychogeriatr. 2010; 22(3):463-9. [DOI:10.1017/S1041610209991323] [PMID]
- Kim TH, Huh Y, Choe JY, Jeong JW, Park JH, Lee SB, et al. Korean version of frontal assessment battery: Psychometric properties and normative data. Dement Geriatr Cogn Disord. 2010; 29(4):363-70. [DOI:10.1159/000297523] [PMID]
- Mok VC, Wong A, Yim P, Fu M, Lam WW, Hui AC, et al. The validity and reliability of chinese frontal assessment battery in evaluating executive dysfunction among Chinese patients with small subcortical infarct. Alzheimer Dis Assoc Disord. 2004; 18(2):68-74. [DOI:10.1097/01.wad.0000126617.54783.7] [PMID]
- Benke T, Karner E, Delazer M. FAB-D: German version of the frontal assessment battery. J Neurol. 2013; 260(8):2066-72. [DOI:10.1007/s00415-013-6929-8] [PMID]
- Appollonio I, Leone M, Isella V, Piamarta F, Consoli T, Villa ML, et al. The Frontal Assessment Battery (FAB): Normative values in an Italian population sample. Neurol Sci. 2005; 26(2):108-16.[DOI:10.1007/s10072-005-0443-4] [PMID]
- Asaadi S, Ashrafi F, Omidbeigi M, Nasiri Z, Pakdaman H, Amini-Harandi A. Persian version of frontal assessment battery: Correlations with formal measures of executive functioning and providing normative data for Persian population. Iran J Neurol. 2016; 15(1):16-22. [PMID]
- Gouvier WD, Blanton PD, LaPorte KK. Reliability and validity of the disability rating scale and the levels of cognitive functioning scale in monitoring recovery from severe head injury. J Head Trauma Rehabil. 1987; 2(4):91. [DOI:10.1097/00001199-198712000-00015]
- Singh S, Aich TK, Bhattarai R. Wisconsin Card Sorting Test performance impairment in schizophrenia: An Indian study report. Indian J Psychiatry. 2017; 59(1):88-93. [DOI:10.4103/0019-5545.204440] [PMID]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test (WCST): Manual: Revised and expanded. Los Angeles: Psychological Assessment Services, Inc; 1993. [Link]
- Greve KW. The WCST-64: A standardized short-form of the Wisconsin Card Sorting Test. Clin Neuropsychol. 2001; 15(2):228-34.[DOI:10.1076/clin.15.2.228.1901] [PMID]
- Rahimi C, Hashemi R, Mohamadi N. The utility of the wisconsin card sorting test in differential diagnosis of cognitive disorders in Iranian psychiatric patients and healthy subjects. Iran J Psychiatry. 2011; 6(3):99-105. [PMID]
- Scarpina F, Tagini S. The stroop color and word test. Front Psychol. 2017; 8:557. [DOI:10.3389/fpsyg.2017.00557] [PMID] [PMCID]
- Aliloo MM, Hamidi S, Shirvani A. [Comparison of executive function and sustained attention in students with obsessive-compulsive, high schizotypal and overlapping symptoms with the normal group (Persian)]. J Res Behav Sci. 2011; 9(3):216-21. [Link]
- Chien CW, Brown T, McDonald R, Rodger S. Convergent and discriminant validity of a naturalistic observational assessment of children’s hand skills. Hong Kong J Occup Ther. 2011; 21(2):64-71. [DOI:10.1016/j.hkjot.2011.10.003]
- Perkins NJ, Schisterman EF. The Youden Index and the optimal cut‐point corrected for measurement error. Biom J. 2005; 47(4):428-41. [DOI:10.1002/bimj.200410133] [PMID]
- Poewe W. Non‐motor symptoms in Parkinson’s disease. Eur J Neurol. 2008; 15(Suppl 1):14-20. [DOI:10.1111/j.1468-1331.2008.02056.x] [PMID]
- Kugo A, Terada S, Ata T, Ido Y, Kado Y, Ishihara T, et al. Japanese version of the Frontal Assessment Battery for dementia. Psychiatry Res. 2007; 153(1):69-75. [DOI:10.1016/j.psychres.2006.04.004] [PMID]
- Gauthier S, LeBlanc J, Seresova A, Laberge-Poirier A, A Correa J, Alturki AY, et al. Acute prediction of outcome and cognitive-communication impairments following traumatic brain injury: The influence of age, education and site of lesion. J Commun Disord. 2018; 73:77-90. [DOI:10.1016/j.jcomdis.2018.04.003] [PMID]
- Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017; 16(12):987-1048. [DOI:10.1016/S1474-4422(17)30371-X] [PMID]
- Slachevsky A, Villalpando JM, Sarazin M, Hahn-Barma V, Pillon B, Dubois B. Frontal assessment battery and differential diagnosis of frontotemporal dementia and Alzheimer disease. Arch Neurol. 2004; 61(7):1104-7. [DOI:10.1001/archneur.61.7.1104] [PMID]
- Oguro H, Yamaguchi S, Abe S, Ishida Y, Bokura H, Kobayashi S. Differentiating Alzheimer’s disease from subcortical vascular dementia with the FAB test. J Neurol. 2006; 253(11):1490-4. [DOI:10.1007/s00415-006-0251-7] [PMID]
- Brück A, Aalto S, Nurmi E, Bergman J, Rinne JO. Cortical 6-[18F] fluoro-L-dopa uptake and frontal cognitive functions in early Parkinson’s disease. Neurobiol Aging. 2005; 26(6):891-8. [DOI:10.1016/j.neurobiolaging.2004.07.014] [PMID]
- Harrison BJ, Shaw M, Yücel M, Purcell R, Brewer WJ, Strother SC, et al. Functional connectivity during Stroop task performance. Neuroimage. 2005; 24(1):181-91. [DOI:10.1016/j.neuroimage.2004.08.033] [PMID]
- Yoshida H, Terada S, Sato S, Kishimoto Y, Ata T, Ohshima E, et al. Frontal assessment battery and brain perfusion imaging in early dementia. Dement Geriatr Cogn Disord. 2009; 27(2):133-8. [DOI:10.1159/000198687] [PMID]
Type of Study: Research |
Subject:
Occupational Therapy
Received: 2024/10/6 | Accepted: 2024/10/20 | Published: 2025/03/22
Received: 2024/10/6 | Accepted: 2024/10/20 | Published: 2025/03/22





