Volume 6, Issue 1 (Continuously Updated 2023)
Func Disabil J 2023, 6(1): 0-0 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ghorbani M, Maarefvand M, Sepehrnejad M. Gap in Noise Auditory Brainstem Responses: A Systematic Review. Func Disabil J 2023; 6 (1) : 262.1
URL: http://fdj.iums.ac.ir/article-1-226-en.html
URL: http://fdj.iums.ac.ir/article-1-226-en.html
1- Department of Audiology, School of Rehabilitation Sciences, Iran University of Medical Sciences, Tehran, Iran. , mtr.ghorbani128@gmail.com
2- Department of Audiology, School of Rehabilitation Sciences, Iran University of Medical Sciences, Tehran, Iran.
3- Department of Audiology, School of Rehabilitation Sciences, Isfahan University of Medical Sciences, Isfahan, Iran.
2- Department of Audiology, School of Rehabilitation Sciences, Iran University of Medical Sciences, Tehran, Iran.
3- Department of Audiology, School of Rehabilitation Sciences, Isfahan University of Medical Sciences, Isfahan, Iran.
Full-Text [PDF 1385 kb]
(425 Downloads)
| Abstract (HTML) (1602 Views)
Full-Text: (680 Views)
Introduction
The auditory brainstem response (ABR) is a test designed to assess the functioning of the auditory brainstem in response to auditory stimuli [1]. It is a simple, objective, and noninvasive procedure to evaluate the brainstem’s cochlear nerve and auditory circuits [2]. The ABR test is used to evaluate suspected neurologic disorders of the eighth cranial nerve and its related auditory pathways, as well as to objectively estimate hearing sensitivity for persons who are unable to offer correct behavioral hearing evaluation information [3].
The gap in noise (GIN) test is a commonly utilized clinical procedure that belongs to the behavioral methods category in the field of clinical psychoacoustic evaluation [4]. However, it is essential to note certain limitations associated with these behavioral approaches and psychoacoustic evaluations. These methods heavily rely on patients’ subjective judgments, which may be influenced by instructions given before and during the testing process. Furthermore, the results obtained may be affected by factors, such as the frequency of test administration. Additionally, individual performance in these tests can be influenced by various factors, including attention, concentration, and motivation [5-7].
The GIN-ABR is a type of auditory brainstem response testing that focuses on detecting gaps in continuous noise signals. This method serves as an objective and non-invasive measure of auditory temporal processing, providing valuable information about the functionality of the auditory system spanning from the cochlea to the brainstem. By analyzing various waveform components of the ABR, GIN-ABR offers insights into both peripheral and brainstem auditory processing abilities when detecting temporal gaps in ongoing sounds [8].
GIN-ABR offers several notable advantages compared to alternative approaches to evaluate auditory temporal processing. These include its non-invasive nature, objective measurement of auditory temporal processing, and comprehensive assessment of the auditory system. This method is valuable to investigate a range of conditions including age-related hearing loss, auditory processing disorders, and tinnitus [4, 8-13]. Furthermore, studies have explored the correlation between gap detection thresholds obtained through ABR testing and behavioral thresholds [10, 11, 14].
GIN-ABR uses a broadband noise stimulus with a silent gap embedded in the noise. To determine the minimal detectable gap duration, researchers manipulated the duration of the gap in stimuli and observed participants’ responses. The GIN-ABR waveform consists of a series of peaks and troughs that correspond to different neural generators in the auditory pathway. The ABR waveform is analyzed to determine the latency and amplitude of each peak and trough, which can be utilized as a means to evaluate the integrity of the auditory processing system [4, 5].
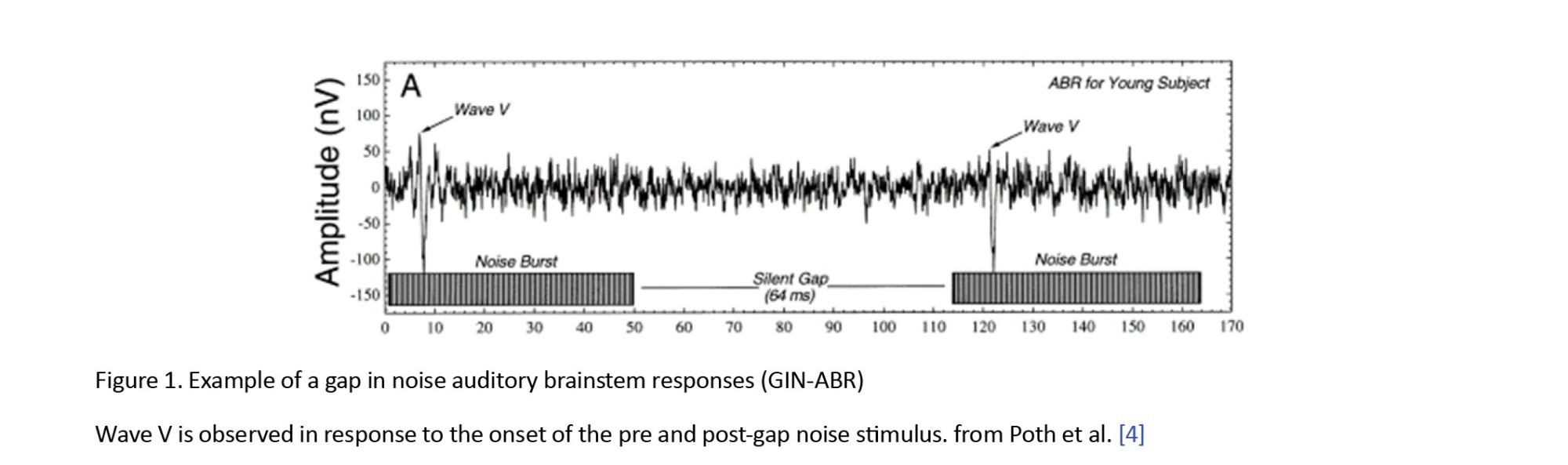
ABR shows a remarkable synchronization with the onset of a stimulus, demonstrating precise alignment both in terms of time and phase. Figure 1 shows this synchronicity, where the presence of a gap in the stimulus gives rise to two distinct points of origin, one preceding the gap (referred to as pre-gap) and another succeeding it (known as post-gap). As a result, due to its close resemblance to the shape and physical characteristics of the stimulus, ABR enables the recording of two separate responses- corresponding to the pre-gap segment and another representing the post-gap segment [13, 14].
This review was conducted to present a thorough examination of the gap in noise auditory brainstem responses, encompassing its methodology, diverse applications, and inherent limitations. Additionally, we address the current state of research on GIN-ABR, focusing particularly on its utilization in exploring the impacts of auditory processing disorders, hearing loss, tinnitus, and age-related factors. Furthermore, we explore potential future avenues for research about GIN-ABR testing and discuss its prospective clinical applications.
Materials and Methods
This study adhered to the guidelines outlined by the preferred reporting items for systematic reviews and meta-analyses (PRISMA) framework. PubMed, Cochrane, and Ovid databases were used to extract data from 1990 up to June 2023. In addition, a manual search was also performed through Google Scholar to find studies that were not identified in the above databases for any reason. The search terms used were “gap in noise auditory brainstem response”, “ABR gap detection”, “ABR gap duration”, “ABR gap threshold”, and “ABR temporal processing”. The keywords used alone or in combination (“and”) and mesh (medical subject headings) terms were searched when available. The search was not limited to human studies and also included animal studies. Letters to the editor, review articles and case reports were not included in the search. The main focus of the research was to investigate gap-evoked auditory brainstem responses in various groups of participants. Gaps, which refer to silent intervals in a stimulus sound, were precisely defined for this purpose and ABR were recorded by pre or post-gap stimulus sections. Articles were included if there was the use of gap-evoked ABR, investigating the effect of any factor on the responses, and using any gapped stimulus type. Articles whose evoked potentials did not include ABR waves were excluded. To ensure the reliability and quality of the included content, non-peer-reviewed sources, such as magazines, conference proceedings, editorials, manuals, and articles in languages other than English were excluded from this study.
The initial phase of the review process entailed screening the titles and abstracts of studies to assess their eligibility based on predetermined inclusion criteria. Subsequently, all selected articles were reviewed in full-text form to further refine the selection and eliminate any studies that did not align with the research objectives. This meticulous evaluation ensured that only relevant and suitable research was included in the final analysis. The chosen studies were then carefully analyzed for their content and organized into a table based on features, such as authors, year of publication, journal and indexing, individual characteristics (e.g. sample size, age, gender, hearing status, species studied), ABR gap in noise stimulus details (noise bandwidth, rise/fall time, duration, filters, gap duration), methods used (e.g. examining onset or offset of gaps, manipulating gap features, employing different gap durations, using various stimulus frequencies), dependent variables measured and major results.
Results
The initial search yielded a total of 1 198 studies using the specified keywords. After removing duplicates, 903 unique studies remained for further evaluation based on the inclusion and exclusion criteria, primarily through assessing their titles and abstracts. Articles were excluded from consideration due to factors, such as the use of electrophysiological tests other than ABR, the use of gapless stimuli, limited availability of full-text access in English, or conference proceedings. Following this screening process, 58 studies met the eligibility criteria and underwent a thorough examination of their full texts. Eventually, after careful analysis, 10 studies were deemed suitable for inclusion in the review. Figure 2 shows the selection process using the preferred reporting items for systematic reviews and meta-analyses (PRISMA) flowchart methodology.
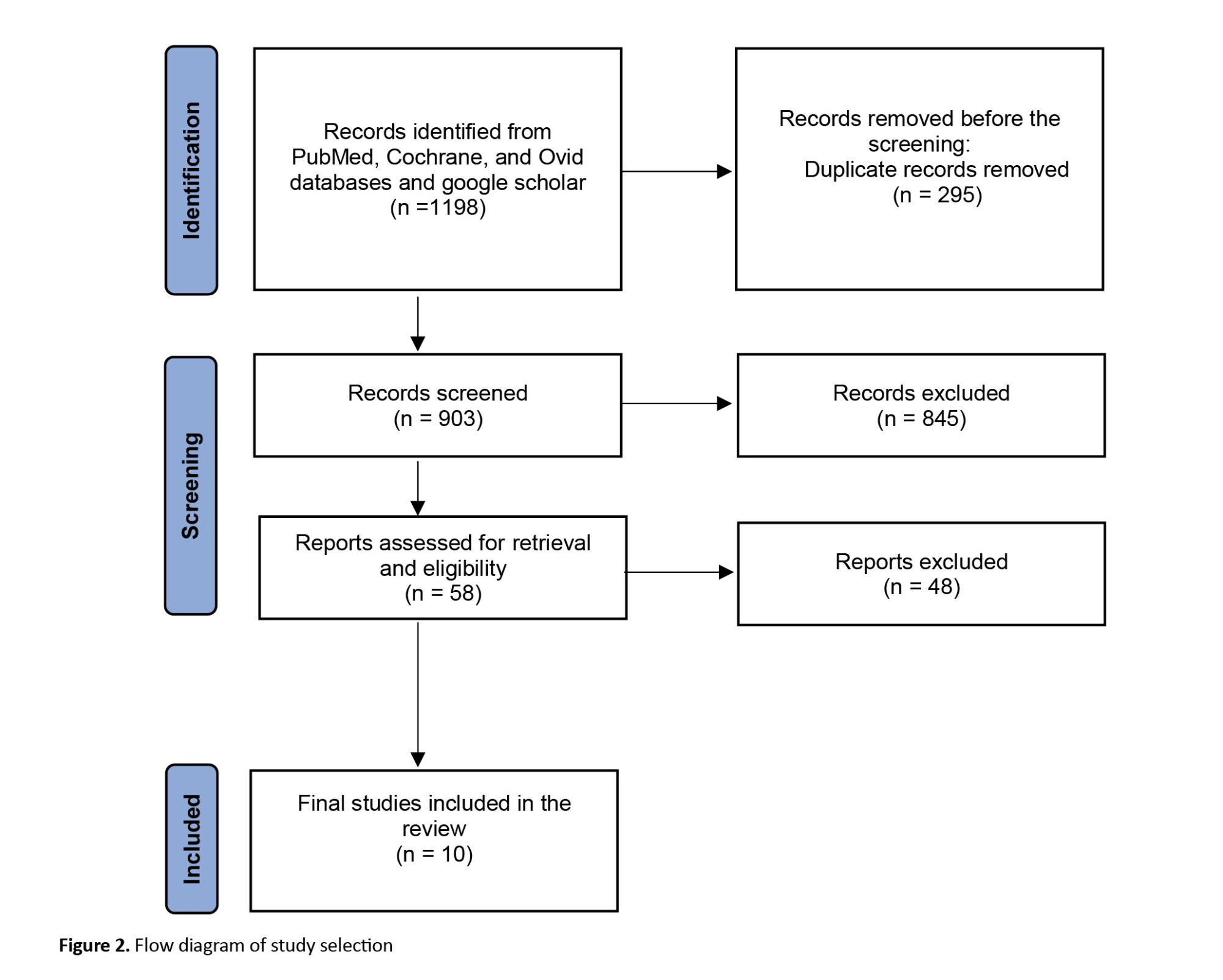
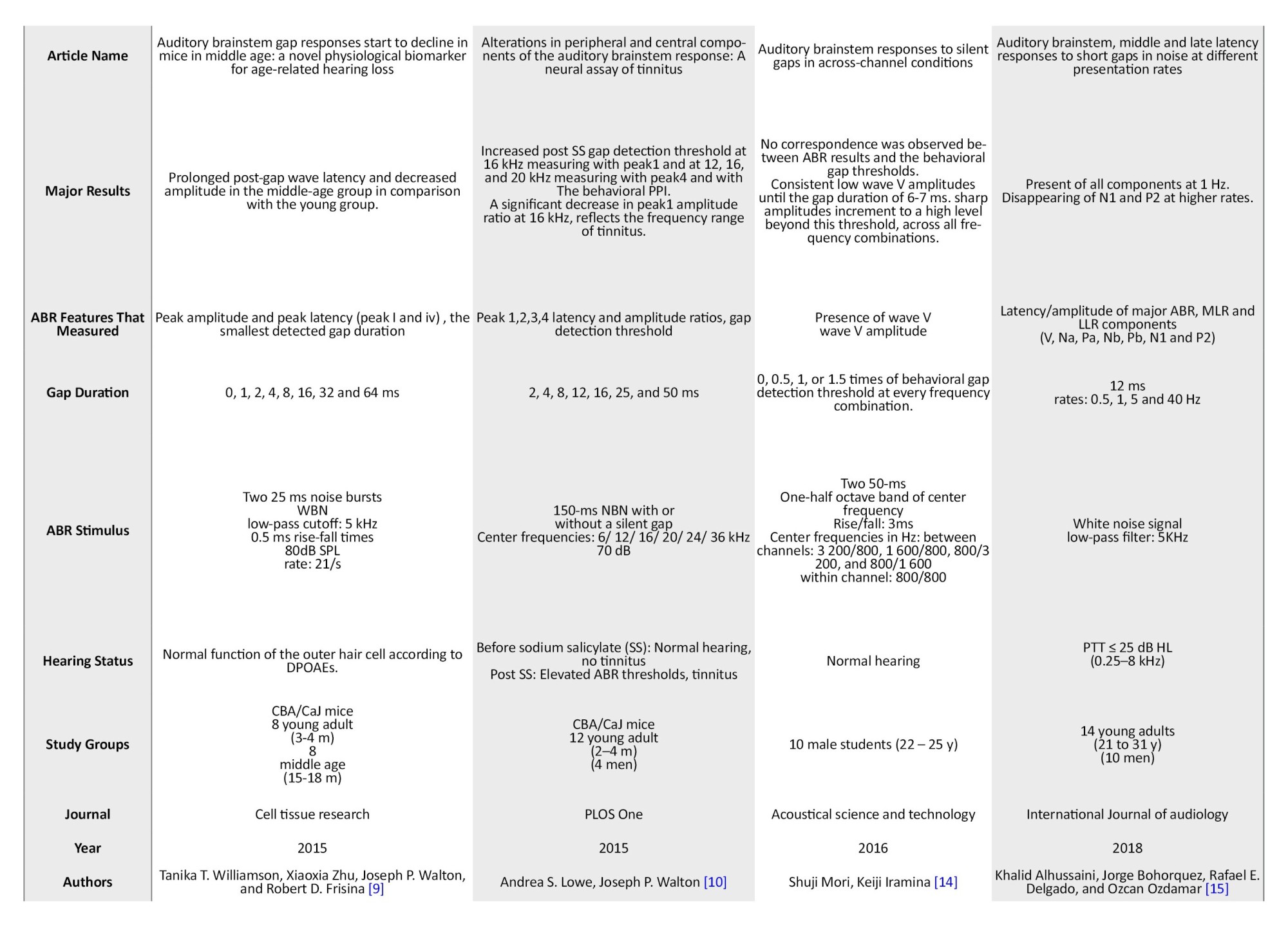
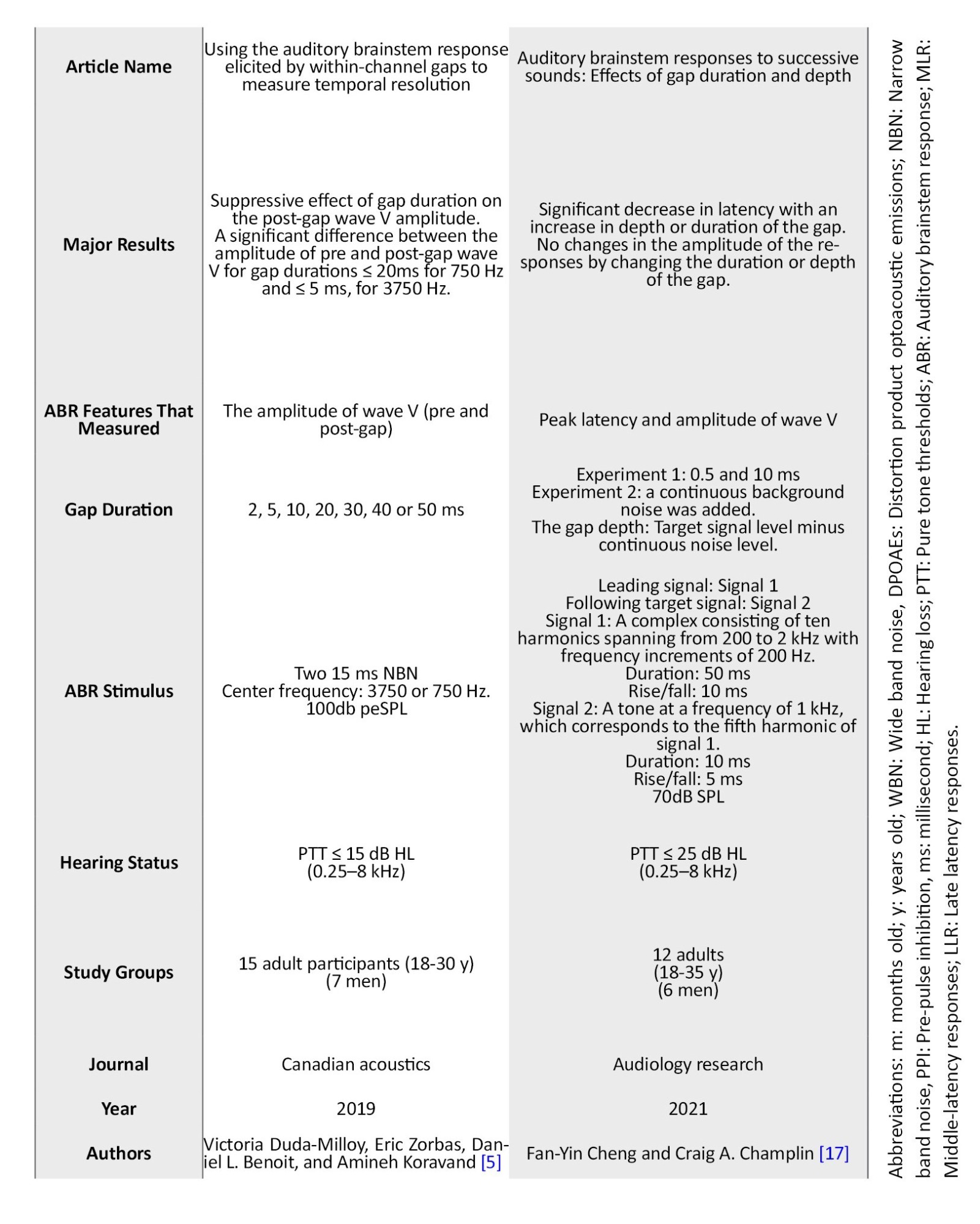
Table 1 presents the summarized information extracted from the final included studies.

Population
Of the 10 articles that were finally reviewed, 7 articles were about people with normal hearing, 3 articles about people with sensory-neural, age-related, or salicylate-induced hearing loss, and 1 article about people with tinnitus. Humans were investigated in 7 studies, and in other studies, the investigation was conducted on animals (1 on Mongolian gerbils and 2 on CBA Carter mice.).
The number of participants in all human studies was 121, of which 44% were men and 56% were women. This number for animal studies was 48 animals. In the human studies, young individuals ranged in age from 18 to 30 years, while the age groups included individuals aged between 58 and 72 years. In animal studies, the reported age range was between 2-4 months in Lowe et al.’s study [10], young (4-8 m) and aged (33-37 m) in Boettcher et al.’s study [12], young adults (3-4 m) and middle age (15-18 m) in Williamson et al.’s study [9].
In human studies, the hearing assessment involves measuring pure tone thresholds. Normal hearing was defined as pure tone thresholds equal to or below 25 dBHL according to Poth et al. and Aluussaini et al. [4, 15], equal to or less than 20 dBHL in Grose et al.’s study [16] and equal to or lower than 15 dBHL in Duda-Milloy et al.’s study at each clinical octave in the range of 250 to 8000 Hz [5]. Also in Fan et al.’s study, the thresholds equal to or lower than 25 dBHL in 250-8000 Hz were considered normal hearing [17]. In Werner et al.’s study, normal hearing status was verified through screening audiometry at octave frequencies from 500 to 8000 Hz, with thresholds set at 15 dBHL. Additionally, click-evoked ABR testing was performed at a level of 20 dB nHL to further confirm the participants’ normal auditory function [11]. In a study, normal hearing was reported, without specifying exact thresholds [14]. Hearing thresholds exceeding 8 kHz were not reported in any human studies. In an animal study conducted by Williamson et al., the functionality of the outer hair cell system was assessed using distortion product otoacoustic emissions (DPOAEs) [9].The study conducted by Lowe et al. described hearing changes as an increment in the average ABR thresholds [10]. Boettcher et al did not mention hearing status measurement protocol [12].
Methods
The human studies investigated the auditory brainstem wave V features, such as absolute peak latency and amplitudes, latency, and amplitudes shift, latency and amplitude ratios. Wave V was recorded in response to pre and post-gap stimulus onsets. The animal studies focused on examining the characteristics of auditory brainstem waves 1 to 4. Most of these studies utilized wideband noises with gaps inserted, employing various filters (n=5). Alternatively, narrowband noises within different frequency bands were used in the remaining studies (n=4), covering frequencies ranging from 750 Hz to 36000 Hz. It is worth noting that human data was only reported up to 4000 Hz. Fan et al used a harmonic complex [17].
In terms of stimulus presentation levels, they typically ranged from 60 to 85 dB sound pressure level (SPL) in three studies, but in two studies, the level varied between 10 to 50 dB SPL (n=1) or was fixed at 100 dB peSPL (n=1). The length of pre and post-gap stimuli was different in studies. Signals with a length of 12 ms (n=1), 15 ms (n=2), 25 ms (n=1), 50 ms (n=4), 100 ms (n=1), and 150 ms (n=1) were used in the studies. In Allhussaini et al. and experiment 2 of Fan et al., gaps of constant length were inserted between two signals, with the gap duration set at 12 ms and 5 ms, respectively [15, 17]. Similarly, in Mori et al.’s study, the gap length was determined individually for each listener based on their gap thresholds. The gap duration used in this study was either identical to the measured threshold or 0.5, 1, or 1.5 times longer than the individual’s gap threshold duration for each frequency combination [14]. Most of the remaining studies used gaps with lengths of 0 t 64 ms in different combinations. Werner et al used 0 to 125 ms gap durations in their study [11].
Seven studies used the gap in noise stimuli in within-channel conditions, meaning that the frequency content of pre and post-gap noise did not differ from each other. However, three studies used between-channel mode. Grose et al. used frequency-asymmetric markers [16]containing a single primary tone with a two-tone trailing marker (2285 Hz, 4000 Hz). In the study conducted by Mori et al., various combinations of center frequencies were used in 800/1600 Hz, 800/3200 Hz, 1600/800 Hz, 3200/800 Hz (between channel), and 800/800 Hz (within channel) [14]. Fan et al stimuli contained two signals. The first signal consisted of ten 200 to 2 kHz harmonics at frequency intervals of 200 Hz. The second signal, referred to as the target signal, was a tone with a frequency of 1000 Hz combined with the fifth harmonic of the first signal [17].
Outcomes
Most studies have investigated and compared the latency and amplitude of ABR waves elicited with the pre and post-gap stimuli. Factors, such as latency shift (the difference of waves latency in response to the pre and post-gap stimulus), and amplitude ratio (the ratio of the amplitude of the pre and post-gap waves) have also been calculated. Studies that have investigated the effect of age-induced hearing changes and age report almost the same results. The latency shift was longer in the aged group compared to the young group [9, 12]. Boetcher et al. reported amplitude ratios with small changes across age groups while Williamson et al. reported that the middle-aged group of mice showed decreased peak amplitude compared to the young group. In the study conducted by Poth et al., older adults showed similar response latencies to younger subjects, but smaller amplitudes were observed. Also, the gap detection thresholds were higher in the aged group in this study [4].
In Lowe et al.’s study a significant reduction in amplitude ratio was observed only near the tinnitus frequency as estimated based on gap pre-pulse inhibition (GPPI) [10]. In the study conducted by Werner et al, a partial correlation (0.39 and P=0.011) was reported between the average electrophysiological threshold (2.4±0.1 ms) and the average psychophysical threshold (2.9±0.2 ms) [11]. In contrast, Mori et al. observed that the onset response of ABR was not consistently consistent with behavioral gap detection thresholds, particularly when considering various between-channel and within-channel conditions. [14].
Duda-Miloy et al. reported that the duration of the gap has a suppressive impact on the wave V amplitude in response to post-gap noise burst [5]. On the other hand, Fan et al. found that neither the duration nor depth of the gap influenced response amplitude; however, they observed a significant decrease in response latency with increasing gap duration or depth [17]. Similarly, Grose et al. reported that in conditions where gaps were within-channel, shorter gap durations resulted in increased wave V latency and deteriorated waveform [16].
Discussion
The GIN-ABR is a relatively novel assessment technique that holds promise for clinical applications. It has been extensively studied across a range of contexts, including age-related hearing loss and tinnitus [4, 9-12]. Additionally, researchers have focused on exploring the correlation between GIN-ABR results and subjective evaluations, as well as investigating the influence of various factors on different components of this test [5, 11, 14-17].
Age
GIN-ABR has been used to investigate the effects of age on auditory temporal processing across different age periods. Two studies showed that the latency of the post-gap ABR waveform increases with age, indicating a decline in the speed of neural processing with age, suggesting that it can be an innovative physiological marker in case of age-related hearing changes even in the very early stages [9, 12]. Also, some other studies found that the post-gap ABR responses start to decline with increasing age [4, 9], and gap detection thresholds increase [4]. The results indicated that age-related gap detection deficits exist at the brainstem level among older individuals without hearing threshold changes [4]. Gap detection thresholds, using ABR, in 3-month-old infants showed no difference from those of adults with normal hearing in Werner et al.'s study [11].
The age-related decline in amplitude levels has been attributed to various factors, such as diminished temporal synchronization among neurons, declined number of responsive neurons, and decreased endocochlear potential [18, 19]. Spiral ganglion (SG) cells, located in the modiolus, send excitatory signals to the central auditory system, particularly to the cochlear nucleus. In this interaction, SG excitatory neurons interact with neurons that utilize inhibitory neurotransmitters, such as glycine or gamma-aminobutyric acid [19, 20]. Age-related reduction in responsive nerve fibers in SG disturbs the balance between inhibition and excitation. This decline in neural coding capabilities within the central auditory system is associated with age-related changes at hair cell/SG neuron synapses and reductions in hair cell and neuron numbers. Consequently, studies have suggested that post-gap ABR peak latency and amplitude are compromised or degraded due to impaired temporal processing and inhibitory functions [9].
Hearing loss
GIN-ABR has been used to investigate the effects of hearing loss on temporal processing, just like the conventional subjective gap in noise tests. In Werner et al’s study, participants with hearing loss exhibited an average psychophysical threshold of 10.7 ms compared to normal hearing participants (2.6 ms). Similarly, the ABR threshold averaged 12.7 ms for those with hearing loss and 2.7 ms for those with normal hearing. These results indicated that sensorineural hearing loss has the same effect on both psychophysical and ABR thresholds, while the psychophysical gap detection threshold is slightly higher than the ABR gap threshold [11]. Studies that have used behavioral and psychophysical gap detection tests also showed that the gap detection threshold is higher in individuals with hearing loss than in individuals with normal hearing, indicating a decline in auditory temporal processing with hearing loss. The mechanisms underlying sensorineural hearing loss seem to impact both behavioral and electrophysiological gap thresholds. This suggests that peripheral factors associated with sensorineural hearing loss limit the ability of individuals with hearing loss to detect gaps. Consequently, ABR may serve as a valuable tool to investigate temporal resolution in individuals with hearing loss [11].
Tinnitus
Lowe et al. found a notable reduction in amplitude ratio (recovery time) specifically around the frequency associated with sodium salicylate (SS)-induced tinnitus, which was measured using the reliable gap pre-pulse inhibition (GPPI) method [10]. This study highlights the effectiveness of GIN-ABRs as a non-invasive approach to detect the presence of tinnitus and or approximate pitch in mouse models objectively. Furthermore, it suggests that GIN-ABR can potentially be applied to human subjects and reveals distinct impairments in temporal processing within peripheral and brainstem pathways, affected in drug-induced tinnitus. In a review article on ABR in tinnitus, GIN-ABR was recognized as one technique capable of objectively assessing the presence of tinnitus [8].
In the studies conducted in recent years, it has been found that people with tinnitus have shown higher thresholds in tests related to temporal processing, such as types of GIN and gap detection tests [10, 21-24]. Contemporary research articles explore the question of whether the elevation in gap detection thresholds observed in individuals with tinnitus is attributed to a general impairment in temporal resolution [21-23, 25] or if tinnitus itself masks the silent gap between two stimulus components [10, 24, 26]. Evidence has been presented supporting both theories, indicating that further investigation is necessary to gain deeper insights into this area of study.
Clinical application
So far, gap in noise has been electrophysiologically investigated using methods, such as ABR, auditory middle latency responses (MLR), event-related potentials (ERP), and mismatch negativity in studies.
As mentioned, GIN-ABR is a method that has been investigated in animal and human studies and in people with normal hearing, sensorineural hearing loss, and tinnitus, as well as in infants and the elderly. The results of these studies have shown the appropriate use of this test in experimental groups. Also, in recent studies, the effect of various factors, such as the frequency of the stimulating noise, gap length, and depth on the amplitude and latency of waves has been investigated [5, 16, 17]. This is a big step towards bringing GIN-ABR closer to clinical application.
In the study of Alhussaini et al., a detailed comparison was made between the characteristics of ABR, mid-range, and late auditory responses in response to GIN stimuli [15]. In this study, the effect of the stimulation presentation rate on these responses was investigated, showing that most of these electrophysiological responses to the gap stimulus in noise can be obtained at a rate of 1 Hz; meanwhile, at higher stimulation presentation rates, only ABR and middle latency responses (MLR) can be recorded, indicating the greater priority of the GIN-ABR test in clinical routine evaluations compared to late responses. This priority can be due to the higher speed and shorter test time.
The use of ABR as a clinical electrophysiological tool for gap detection has other advantages. Among them, we can mention non-invasiveness, cost-effectiveness, and the possibility of using a large number of audiologists [5]. Meanwhile, providing the necessary environment, equipment, expertise and experience for the implementation of event-related potentials (ERP) tests in clinics will not be easy. In addition, unlike late responses and other electrophysiological tests of hearing, ABR is less affected by alertness, depth of sleep, and medications taken by people [27]. Another advantage of using electrophysiological GIN compared to studies that investigate conventional electrophysiological tests to find an objective clinical evaluation of hearing system function is that the effect of complications on the amplitude or latency of waves in electrophysiological GIN is eliminated. Thus, any potential effects of interindividual variation on amplitude, waveform, latency, or other characteristics of waves is replicated twice, pre-gap and post-gap. As a result, the possibility of interfering with the purpose of the test, which is the gap detection threshold, is reduced.
To develop electrophysiological tests in line with behavioral gap detection tests, studies have been conducted that behavioral GIN thresholds in various groups of patients are closely related to gap detection thresholds in noise, which are determined by GIN-ABR [11, 28]. Also, The GIN-ABR holds promise in providing frequency-specific information to investigate temporal resolution in individuals with diverse hearing disorders [5].
5. Conclusion
The GIN-ABR is a valuable assessment tool for auditory temporal processing, offering non-invasive and objective measurements that encompass the entire auditory system from the cochlea to the brainstem. This method has been extensively utilized in investigating various factors, including age-related effects, hearing loss, tinnitus, and auditory processing disorders. Notably, GIN-ABR has several clinical advantages, such as cost-effectiveness, shorter test duration compared to other electrophysiological methods to assess gaps in noise perception, independence from alertness levels, attention span variations, sleep depth fluctuations, or medication influences commonly encountered among individuals. Additionally, it has been shown to be compatible with psychophysical tests.
Despite its strengths and applications, it is essential to acknowledge certain limitations associated with GIN-ABR that warrant careful interpretation of results. Ongoing research efforts are needed to comprehensively explore the potential clinical implications of this technique and elucidate its precise role within the broader context of evaluating auditory system function. Further deepening our understanding of these aspects through rigorous investigation and studies conducted on larger populations across diverse settings and conditions ultimately enhances our ability to leverage GIN-ABR effectively for diagnostic purposes and treatment planning.
Limitations
GIN-ABR has limitations that should be considered when interpreting the results. One limitation is that GIN-ABR only measures auditory temporal processing up to the brainstem. Other auditory temporal processing measurement tools, such as gap detection using behavioral measures, can evaluate higher levels of the auditory temporal processing system. Another limitation is that GIN-ABR uses a broadband noise stimulus, which may not be representative of real-world listening situations. Finally, GIN-ABR requires specialized equipment and expertise, which may limit its availability in some clinical settings.
Future directions
Future research on GIN-ABR may focus on developing new methods of data analysis to improve the accuracy and reliability of the results. For example, machine learning algorithms may be used to analyze the GIN-ABR waveform to identify patterns that are associated with specific auditory disorders or use frequency-specific stimuli to obtain detailed information from different parts of the auditory pathway by region. Future research may also focus on developing new applications of GIN-ABR testing, such as using it to assess the efficacy of hearing loss treatments or to predict the risk of developing auditory disorders before the onset of clinical symptoms.
Ethical Considerations
Compliance with ethical guidelines
This research did not involve the use of human volunteers or animals, ensuring compliance with ethical guidelines.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the Audiology Department of Iran and Isfahan Universities of Medical Sciences for their cooperation and help in designing the study.
References
The auditory brainstem response (ABR) is a test designed to assess the functioning of the auditory brainstem in response to auditory stimuli [1]. It is a simple, objective, and noninvasive procedure to evaluate the brainstem’s cochlear nerve and auditory circuits [2]. The ABR test is used to evaluate suspected neurologic disorders of the eighth cranial nerve and its related auditory pathways, as well as to objectively estimate hearing sensitivity for persons who are unable to offer correct behavioral hearing evaluation information [3].
The gap in noise (GIN) test is a commonly utilized clinical procedure that belongs to the behavioral methods category in the field of clinical psychoacoustic evaluation [4]. However, it is essential to note certain limitations associated with these behavioral approaches and psychoacoustic evaluations. These methods heavily rely on patients’ subjective judgments, which may be influenced by instructions given before and during the testing process. Furthermore, the results obtained may be affected by factors, such as the frequency of test administration. Additionally, individual performance in these tests can be influenced by various factors, including attention, concentration, and motivation [5-7].
The GIN-ABR is a type of auditory brainstem response testing that focuses on detecting gaps in continuous noise signals. This method serves as an objective and non-invasive measure of auditory temporal processing, providing valuable information about the functionality of the auditory system spanning from the cochlea to the brainstem. By analyzing various waveform components of the ABR, GIN-ABR offers insights into both peripheral and brainstem auditory processing abilities when detecting temporal gaps in ongoing sounds [8].
GIN-ABR offers several notable advantages compared to alternative approaches to evaluate auditory temporal processing. These include its non-invasive nature, objective measurement of auditory temporal processing, and comprehensive assessment of the auditory system. This method is valuable to investigate a range of conditions including age-related hearing loss, auditory processing disorders, and tinnitus [4, 8-13]. Furthermore, studies have explored the correlation between gap detection thresholds obtained through ABR testing and behavioral thresholds [10, 11, 14].
GIN-ABR uses a broadband noise stimulus with a silent gap embedded in the noise. To determine the minimal detectable gap duration, researchers manipulated the duration of the gap in stimuli and observed participants’ responses. The GIN-ABR waveform consists of a series of peaks and troughs that correspond to different neural generators in the auditory pathway. The ABR waveform is analyzed to determine the latency and amplitude of each peak and trough, which can be utilized as a means to evaluate the integrity of the auditory processing system [4, 5].
ABR shows a remarkable synchronization with the onset of a stimulus, demonstrating precise alignment both in terms of time and phase. Figure 1 shows this synchronicity, where the presence of a gap in the stimulus gives rise to two distinct points of origin, one preceding the gap (referred to as pre-gap) and another succeeding it (known as post-gap). As a result, due to its close resemblance to the shape and physical characteristics of the stimulus, ABR enables the recording of two separate responses- corresponding to the pre-gap segment and another representing the post-gap segment [13, 14].

This review was conducted to present a thorough examination of the gap in noise auditory brainstem responses, encompassing its methodology, diverse applications, and inherent limitations. Additionally, we address the current state of research on GIN-ABR, focusing particularly on its utilization in exploring the impacts of auditory processing disorders, hearing loss, tinnitus, and age-related factors. Furthermore, we explore potential future avenues for research about GIN-ABR testing and discuss its prospective clinical applications.
Materials and Methods
This study adhered to the guidelines outlined by the preferred reporting items for systematic reviews and meta-analyses (PRISMA) framework. PubMed, Cochrane, and Ovid databases were used to extract data from 1990 up to June 2023. In addition, a manual search was also performed through Google Scholar to find studies that were not identified in the above databases for any reason. The search terms used were “gap in noise auditory brainstem response”, “ABR gap detection”, “ABR gap duration”, “ABR gap threshold”, and “ABR temporal processing”. The keywords used alone or in combination (“and”) and mesh (medical subject headings) terms were searched when available. The search was not limited to human studies and also included animal studies. Letters to the editor, review articles and case reports were not included in the search. The main focus of the research was to investigate gap-evoked auditory brainstem responses in various groups of participants. Gaps, which refer to silent intervals in a stimulus sound, were precisely defined for this purpose and ABR were recorded by pre or post-gap stimulus sections. Articles were included if there was the use of gap-evoked ABR, investigating the effect of any factor on the responses, and using any gapped stimulus type. Articles whose evoked potentials did not include ABR waves were excluded. To ensure the reliability and quality of the included content, non-peer-reviewed sources, such as magazines, conference proceedings, editorials, manuals, and articles in languages other than English were excluded from this study.
The initial phase of the review process entailed screening the titles and abstracts of studies to assess their eligibility based on predetermined inclusion criteria. Subsequently, all selected articles were reviewed in full-text form to further refine the selection and eliminate any studies that did not align with the research objectives. This meticulous evaluation ensured that only relevant and suitable research was included in the final analysis. The chosen studies were then carefully analyzed for their content and organized into a table based on features, such as authors, year of publication, journal and indexing, individual characteristics (e.g. sample size, age, gender, hearing status, species studied), ABR gap in noise stimulus details (noise bandwidth, rise/fall time, duration, filters, gap duration), methods used (e.g. examining onset or offset of gaps, manipulating gap features, employing different gap durations, using various stimulus frequencies), dependent variables measured and major results.
Results
The initial search yielded a total of 1 198 studies using the specified keywords. After removing duplicates, 903 unique studies remained for further evaluation based on the inclusion and exclusion criteria, primarily through assessing their titles and abstracts. Articles were excluded from consideration due to factors, such as the use of electrophysiological tests other than ABR, the use of gapless stimuli, limited availability of full-text access in English, or conference proceedings. Following this screening process, 58 studies met the eligibility criteria and underwent a thorough examination of their full texts. Eventually, after careful analysis, 10 studies were deemed suitable for inclusion in the review. Figure 2 shows the selection process using the preferred reporting items for systematic reviews and meta-analyses (PRISMA) flowchart methodology.

Table 1 presents the summarized information extracted from the final included studies.



Population
Of the 10 articles that were finally reviewed, 7 articles were about people with normal hearing, 3 articles about people with sensory-neural, age-related, or salicylate-induced hearing loss, and 1 article about people with tinnitus. Humans were investigated in 7 studies, and in other studies, the investigation was conducted on animals (1 on Mongolian gerbils and 2 on CBA Carter mice.).
The number of participants in all human studies was 121, of which 44% were men and 56% were women. This number for animal studies was 48 animals. In the human studies, young individuals ranged in age from 18 to 30 years, while the age groups included individuals aged between 58 and 72 years. In animal studies, the reported age range was between 2-4 months in Lowe et al.’s study [10], young (4-8 m) and aged (33-37 m) in Boettcher et al.’s study [12], young adults (3-4 m) and middle age (15-18 m) in Williamson et al.’s study [9].
In human studies, the hearing assessment involves measuring pure tone thresholds. Normal hearing was defined as pure tone thresholds equal to or below 25 dBHL according to Poth et al. and Aluussaini et al. [4, 15], equal to or less than 20 dBHL in Grose et al.’s study [16] and equal to or lower than 15 dBHL in Duda-Milloy et al.’s study at each clinical octave in the range of 250 to 8000 Hz [5]. Also in Fan et al.’s study, the thresholds equal to or lower than 25 dBHL in 250-8000 Hz were considered normal hearing [17]. In Werner et al.’s study, normal hearing status was verified through screening audiometry at octave frequencies from 500 to 8000 Hz, with thresholds set at 15 dBHL. Additionally, click-evoked ABR testing was performed at a level of 20 dB nHL to further confirm the participants’ normal auditory function [11]. In a study, normal hearing was reported, without specifying exact thresholds [14]. Hearing thresholds exceeding 8 kHz were not reported in any human studies. In an animal study conducted by Williamson et al., the functionality of the outer hair cell system was assessed using distortion product otoacoustic emissions (DPOAEs) [9].The study conducted by Lowe et al. described hearing changes as an increment in the average ABR thresholds [10]. Boettcher et al did not mention hearing status measurement protocol [12].
Methods
The human studies investigated the auditory brainstem wave V features, such as absolute peak latency and amplitudes, latency, and amplitudes shift, latency and amplitude ratios. Wave V was recorded in response to pre and post-gap stimulus onsets. The animal studies focused on examining the characteristics of auditory brainstem waves 1 to 4. Most of these studies utilized wideband noises with gaps inserted, employing various filters (n=5). Alternatively, narrowband noises within different frequency bands were used in the remaining studies (n=4), covering frequencies ranging from 750 Hz to 36000 Hz. It is worth noting that human data was only reported up to 4000 Hz. Fan et al used a harmonic complex [17].
In terms of stimulus presentation levels, they typically ranged from 60 to 85 dB sound pressure level (SPL) in three studies, but in two studies, the level varied between 10 to 50 dB SPL (n=1) or was fixed at 100 dB peSPL (n=1). The length of pre and post-gap stimuli was different in studies. Signals with a length of 12 ms (n=1), 15 ms (n=2), 25 ms (n=1), 50 ms (n=4), 100 ms (n=1), and 150 ms (n=1) were used in the studies. In Allhussaini et al. and experiment 2 of Fan et al., gaps of constant length were inserted between two signals, with the gap duration set at 12 ms and 5 ms, respectively [15, 17]. Similarly, in Mori et al.’s study, the gap length was determined individually for each listener based on their gap thresholds. The gap duration used in this study was either identical to the measured threshold or 0.5, 1, or 1.5 times longer than the individual’s gap threshold duration for each frequency combination [14]. Most of the remaining studies used gaps with lengths of 0 t 64 ms in different combinations. Werner et al used 0 to 125 ms gap durations in their study [11].
Seven studies used the gap in noise stimuli in within-channel conditions, meaning that the frequency content of pre and post-gap noise did not differ from each other. However, three studies used between-channel mode. Grose et al. used frequency-asymmetric markers [16]containing a single primary tone with a two-tone trailing marker (2285 Hz, 4000 Hz). In the study conducted by Mori et al., various combinations of center frequencies were used in 800/1600 Hz, 800/3200 Hz, 1600/800 Hz, 3200/800 Hz (between channel), and 800/800 Hz (within channel) [14]. Fan et al stimuli contained two signals. The first signal consisted of ten 200 to 2 kHz harmonics at frequency intervals of 200 Hz. The second signal, referred to as the target signal, was a tone with a frequency of 1000 Hz combined with the fifth harmonic of the first signal [17].
Outcomes
Most studies have investigated and compared the latency and amplitude of ABR waves elicited with the pre and post-gap stimuli. Factors, such as latency shift (the difference of waves latency in response to the pre and post-gap stimulus), and amplitude ratio (the ratio of the amplitude of the pre and post-gap waves) have also been calculated. Studies that have investigated the effect of age-induced hearing changes and age report almost the same results. The latency shift was longer in the aged group compared to the young group [9, 12]. Boetcher et al. reported amplitude ratios with small changes across age groups while Williamson et al. reported that the middle-aged group of mice showed decreased peak amplitude compared to the young group. In the study conducted by Poth et al., older adults showed similar response latencies to younger subjects, but smaller amplitudes were observed. Also, the gap detection thresholds were higher in the aged group in this study [4].
In Lowe et al.’s study a significant reduction in amplitude ratio was observed only near the tinnitus frequency as estimated based on gap pre-pulse inhibition (GPPI) [10]. In the study conducted by Werner et al, a partial correlation (0.39 and P=0.011) was reported between the average electrophysiological threshold (2.4±0.1 ms) and the average psychophysical threshold (2.9±0.2 ms) [11]. In contrast, Mori et al. observed that the onset response of ABR was not consistently consistent with behavioral gap detection thresholds, particularly when considering various between-channel and within-channel conditions. [14].
Duda-Miloy et al. reported that the duration of the gap has a suppressive impact on the wave V amplitude in response to post-gap noise burst [5]. On the other hand, Fan et al. found that neither the duration nor depth of the gap influenced response amplitude; however, they observed a significant decrease in response latency with increasing gap duration or depth [17]. Similarly, Grose et al. reported that in conditions where gaps were within-channel, shorter gap durations resulted in increased wave V latency and deteriorated waveform [16].
Discussion
The GIN-ABR is a relatively novel assessment technique that holds promise for clinical applications. It has been extensively studied across a range of contexts, including age-related hearing loss and tinnitus [4, 9-12]. Additionally, researchers have focused on exploring the correlation between GIN-ABR results and subjective evaluations, as well as investigating the influence of various factors on different components of this test [5, 11, 14-17].
Age
GIN-ABR has been used to investigate the effects of age on auditory temporal processing across different age periods. Two studies showed that the latency of the post-gap ABR waveform increases with age, indicating a decline in the speed of neural processing with age, suggesting that it can be an innovative physiological marker in case of age-related hearing changes even in the very early stages [9, 12]. Also, some other studies found that the post-gap ABR responses start to decline with increasing age [4, 9], and gap detection thresholds increase [4]. The results indicated that age-related gap detection deficits exist at the brainstem level among older individuals without hearing threshold changes [4]. Gap detection thresholds, using ABR, in 3-month-old infants showed no difference from those of adults with normal hearing in Werner et al.'s study [11].
The age-related decline in amplitude levels has been attributed to various factors, such as diminished temporal synchronization among neurons, declined number of responsive neurons, and decreased endocochlear potential [18, 19]. Spiral ganglion (SG) cells, located in the modiolus, send excitatory signals to the central auditory system, particularly to the cochlear nucleus. In this interaction, SG excitatory neurons interact with neurons that utilize inhibitory neurotransmitters, such as glycine or gamma-aminobutyric acid [19, 20]. Age-related reduction in responsive nerve fibers in SG disturbs the balance between inhibition and excitation. This decline in neural coding capabilities within the central auditory system is associated with age-related changes at hair cell/SG neuron synapses and reductions in hair cell and neuron numbers. Consequently, studies have suggested that post-gap ABR peak latency and amplitude are compromised or degraded due to impaired temporal processing and inhibitory functions [9].
Hearing loss
GIN-ABR has been used to investigate the effects of hearing loss on temporal processing, just like the conventional subjective gap in noise tests. In Werner et al’s study, participants with hearing loss exhibited an average psychophysical threshold of 10.7 ms compared to normal hearing participants (2.6 ms). Similarly, the ABR threshold averaged 12.7 ms for those with hearing loss and 2.7 ms for those with normal hearing. These results indicated that sensorineural hearing loss has the same effect on both psychophysical and ABR thresholds, while the psychophysical gap detection threshold is slightly higher than the ABR gap threshold [11]. Studies that have used behavioral and psychophysical gap detection tests also showed that the gap detection threshold is higher in individuals with hearing loss than in individuals with normal hearing, indicating a decline in auditory temporal processing with hearing loss. The mechanisms underlying sensorineural hearing loss seem to impact both behavioral and electrophysiological gap thresholds. This suggests that peripheral factors associated with sensorineural hearing loss limit the ability of individuals with hearing loss to detect gaps. Consequently, ABR may serve as a valuable tool to investigate temporal resolution in individuals with hearing loss [11].
Tinnitus
Lowe et al. found a notable reduction in amplitude ratio (recovery time) specifically around the frequency associated with sodium salicylate (SS)-induced tinnitus, which was measured using the reliable gap pre-pulse inhibition (GPPI) method [10]. This study highlights the effectiveness of GIN-ABRs as a non-invasive approach to detect the presence of tinnitus and or approximate pitch in mouse models objectively. Furthermore, it suggests that GIN-ABR can potentially be applied to human subjects and reveals distinct impairments in temporal processing within peripheral and brainstem pathways, affected in drug-induced tinnitus. In a review article on ABR in tinnitus, GIN-ABR was recognized as one technique capable of objectively assessing the presence of tinnitus [8].
In the studies conducted in recent years, it has been found that people with tinnitus have shown higher thresholds in tests related to temporal processing, such as types of GIN and gap detection tests [10, 21-24]. Contemporary research articles explore the question of whether the elevation in gap detection thresholds observed in individuals with tinnitus is attributed to a general impairment in temporal resolution [21-23, 25] or if tinnitus itself masks the silent gap between two stimulus components [10, 24, 26]. Evidence has been presented supporting both theories, indicating that further investigation is necessary to gain deeper insights into this area of study.
Clinical application
So far, gap in noise has been electrophysiologically investigated using methods, such as ABR, auditory middle latency responses (MLR), event-related potentials (ERP), and mismatch negativity in studies.
As mentioned, GIN-ABR is a method that has been investigated in animal and human studies and in people with normal hearing, sensorineural hearing loss, and tinnitus, as well as in infants and the elderly. The results of these studies have shown the appropriate use of this test in experimental groups. Also, in recent studies, the effect of various factors, such as the frequency of the stimulating noise, gap length, and depth on the amplitude and latency of waves has been investigated [5, 16, 17]. This is a big step towards bringing GIN-ABR closer to clinical application.
In the study of Alhussaini et al., a detailed comparison was made between the characteristics of ABR, mid-range, and late auditory responses in response to GIN stimuli [15]. In this study, the effect of the stimulation presentation rate on these responses was investigated, showing that most of these electrophysiological responses to the gap stimulus in noise can be obtained at a rate of 1 Hz; meanwhile, at higher stimulation presentation rates, only ABR and middle latency responses (MLR) can be recorded, indicating the greater priority of the GIN-ABR test in clinical routine evaluations compared to late responses. This priority can be due to the higher speed and shorter test time.
The use of ABR as a clinical electrophysiological tool for gap detection has other advantages. Among them, we can mention non-invasiveness, cost-effectiveness, and the possibility of using a large number of audiologists [5]. Meanwhile, providing the necessary environment, equipment, expertise and experience for the implementation of event-related potentials (ERP) tests in clinics will not be easy. In addition, unlike late responses and other electrophysiological tests of hearing, ABR is less affected by alertness, depth of sleep, and medications taken by people [27]. Another advantage of using electrophysiological GIN compared to studies that investigate conventional electrophysiological tests to find an objective clinical evaluation of hearing system function is that the effect of complications on the amplitude or latency of waves in electrophysiological GIN is eliminated. Thus, any potential effects of interindividual variation on amplitude, waveform, latency, or other characteristics of waves is replicated twice, pre-gap and post-gap. As a result, the possibility of interfering with the purpose of the test, which is the gap detection threshold, is reduced.
To develop electrophysiological tests in line with behavioral gap detection tests, studies have been conducted that behavioral GIN thresholds in various groups of patients are closely related to gap detection thresholds in noise, which are determined by GIN-ABR [11, 28]. Also, The GIN-ABR holds promise in providing frequency-specific information to investigate temporal resolution in individuals with diverse hearing disorders [5].
5. Conclusion
The GIN-ABR is a valuable assessment tool for auditory temporal processing, offering non-invasive and objective measurements that encompass the entire auditory system from the cochlea to the brainstem. This method has been extensively utilized in investigating various factors, including age-related effects, hearing loss, tinnitus, and auditory processing disorders. Notably, GIN-ABR has several clinical advantages, such as cost-effectiveness, shorter test duration compared to other electrophysiological methods to assess gaps in noise perception, independence from alertness levels, attention span variations, sleep depth fluctuations, or medication influences commonly encountered among individuals. Additionally, it has been shown to be compatible with psychophysical tests.
Despite its strengths and applications, it is essential to acknowledge certain limitations associated with GIN-ABR that warrant careful interpretation of results. Ongoing research efforts are needed to comprehensively explore the potential clinical implications of this technique and elucidate its precise role within the broader context of evaluating auditory system function. Further deepening our understanding of these aspects through rigorous investigation and studies conducted on larger populations across diverse settings and conditions ultimately enhances our ability to leverage GIN-ABR effectively for diagnostic purposes and treatment planning.
Limitations
GIN-ABR has limitations that should be considered when interpreting the results. One limitation is that GIN-ABR only measures auditory temporal processing up to the brainstem. Other auditory temporal processing measurement tools, such as gap detection using behavioral measures, can evaluate higher levels of the auditory temporal processing system. Another limitation is that GIN-ABR uses a broadband noise stimulus, which may not be representative of real-world listening situations. Finally, GIN-ABR requires specialized equipment and expertise, which may limit its availability in some clinical settings.
Future directions
Future research on GIN-ABR may focus on developing new methods of data analysis to improve the accuracy and reliability of the results. For example, machine learning algorithms may be used to analyze the GIN-ABR waveform to identify patterns that are associated with specific auditory disorders or use frequency-specific stimuli to obtain detailed information from different parts of the auditory pathway by region. Future research may also focus on developing new applications of GIN-ABR testing, such as using it to assess the efficacy of hearing loss treatments or to predict the risk of developing auditory disorders before the onset of clinical symptoms.
Ethical Considerations
Compliance with ethical guidelines
This research did not involve the use of human volunteers or animals, ensuring compliance with ethical guidelines.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the Audiology Department of Iran and Isfahan Universities of Medical Sciences for their cooperation and help in designing the study.
References
- Thigpen NN, Keil A. Event-related potentials. In: Reference module in neuroscience and biobehavioral psychology. Amsterdam: Elsevier;2017. [DOI:10.1016/B978-0-12-809324-5.02456-1]
- American Academy of Pediatrics, Joint Committee on Infant Hearing. Year 2007 position statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007; 120(4):898-921. [DOI:10.1542/peds.2007-2333] [PMID]
- Katz J. Handbook of clinical audiology. Buffalo: Lippincott William &Wilkins; 2015. [Link]
- Poth EA, Boettcher FA, Mills JH, Dubno JR. Auditory brainstem responses in younger and older adults for broadband noises separated by a silent gap. Hear Res. 2001;161(1-2):81-6. [DOI:10.1016/S0378-5955(01)00352-5] [PMID]
- Duda-Milloy V, Zorbas E, Benoit DL, Koravand A. Using the auditory brainstem response elicited by within-channel gaps to measure temporal resolution. Can Acoust. 2019; 47(2 ):15-22. [Link]
- Wightman F, Allen P, Dolan T, Kistler D, Jamieson D. Temporal resolution in children. Child Dev. 1989; 60(3):611-24.[DOI:10.2307/1130727] [PMID]
- Green DM. Stimulus selection in adaptive psychophysical procedures. J Acoust Soc Am. 1990; 87(6):2662-74. [DOI:10.1121/1.399058] [PMID]
- Milloy V, Fournier P, Benoit D, Noreña A, Koravand A. Auditory brainstem responses in tinnitus : A review of who , how , and what ? Front Aging Neurosci. 2017;9:237. [DOI:10.3389/fnagi.2017.00237] [PMID]
- Williamson TT, Zhu X, Walton JP, Frisina RD. Auditory brainstem gap responses start to decline in mice in middle age: A novel physiological biomarker for age-related hearing loss. Cell Tissue Res. 2015; 361(1):359-69. [DOI:10.1007/s00441-014-2003-9] [PMID]
- Lowe AS, Walton JP. Alterations in peripheral and central components of the auditory brainstem response: A neural assay of tinnitus. Plos One. 2015; 10(2):e0117228. [DOI:10.1371/journal.pone.0117228] [PMID]
- Werner LA, Folsom RC, Mancl LR, Syapin CL. Human auditory brainstem response to temporal gaps in noise. J Speech Lang Hear Res. 2001; 44(4):737-50. [DOI:10.1044/1092-4388(2001/058)] [PMID]
- Boettcher FA, Mills JH, Swerdloff JL, Holley BL. Auditory evoked potentials in aged gerbils: Responses elicited by noises separated by a silent gap. Hear Res. 1996; 102(1-2):167-78. [DOI:10.1016/S0378-5955(96)90016-7] [PMID]
- Alhussaini K. Analysis of auditory evoked responses elicited by gaps in noise [PhD dissertation]. Florida: University of Miami; 2017. [Link]
- Mori S, Iramina K. Auditory brainstem responses to silent gaps in across-channel conditions. Acoust Sci Technol. 2016; 37(2):79-82. [DOI:10.1250/ast.37.79]
- Alhussaini K, Bohorquez J, Delgado RE, Ozdamar O. Auditory brainstem, middle and late latency responses to short gaps in noise at different presentation rates.Int J Audiol. 2018; 57(6):399-406. [DOI:10.1080/14992027.2018.1428373] [PMID]
- Grose JH, Hall JW, Buss E. Gap duration discrimination for frequency-asymmetric gap markers: Psychophysical and electrophysiological findings. J Acoust Soc Am. 2007; 122(1):446-57. [DOI:10.1121/1.2735106] [PMID]
- Cheng FY, Champlin CA. Auditory brainstem responses to successive sounds: Effects of gap duration and depth. Audiol Res. 2021; 11(1):38-46. [DOI:10.3390/audiolres11010005] [PMID]
- Boettcher FA. Presbyacusis and the auditory brainstem response.J Speech Lang Hear Res. 2002; 45(6):1249-61. [DOI:10.1044/1092-4388(2002/100)] [PMID]
- Khullar S, Babbar R. Presbycusis and auditory brainstem responses: A review. Asian Pac J Trop Dis. 2011; 1(2):150-7. [DOI:10.1016/S2222-1808(11)60056-X]
- Lee HJ, Wallani T, Mendelson JR. Temporal processing speed in the inferior colliculus of young and aged rats. Hear Res. 2002; 174(1-2):64-74. [DOI:10.1016/S0378-5955(02)00639-1] [PMID]
- Mehdizade Gilani V, Ruzbahani M, Mahdi P, Amali A, Nilforush Khoshk MH, Sameni J, et al. Temporal processing evaluation in tinnitus patients: Results on analysis of gap in noise and duration pattern test. Iran J Otorhinolaryngol. 2013; 25(73):221-6. [PMID]
- Sanches SG, Sanchez TG, Carvallo RM. Influence of cochlear function on auditory temporal resolution in tinnitus patients. Audiol Neurootol. 2010; 15(5):273-81. [DOI:10.1159/000272939] [PMID]
- Sanches SG, Samelli AG, Nishiyama AK, Sanchez TG, Carvallo RM. GIN Test (Gaps-in-Noise) in normal listeners with and without tinnitus. Pro Fono. 2010; 22(3):257-62. [DOI:10.1590/S0104-56872010000300017] [PMID]
- Turner JG, Parrish J. Gap detection methods for assessing salicylate-induced tinnitus and hyperacusis in rats. Am J Audiol. 2008; 17(2):S185-92. [DOI:10.1044/1059-0889(2008/08-0006)] [PMID]
- Moon IJ, Won JH, Kang HW, Kim DH, An YH, Shim HJ. Influence of tinnitus on auditory spectral and temporal resolution and speech perception in tinnitus patients. J Neurosci. 2015; 35(42):14260-9. [DOI:10.1523/JNEUROSCI.5091-14.2015] [PMID]
- Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LF, et al. Gap detection deficits in rats with tinnitus: A potential novel screening tool. Behav Neurosci. 2006; 120(1):188-95. [DOI:10.1037/0735-7044.120.1.188] [PMID]
- Fan S, Li S. Objective detection of tinnitus based on electrophysiology. Brain Sci. 2022; 12(8):1086. [DOI:10.3390/brainsci12081086] [PMID]
- Alhussaini K, Bohorquez J, Holt F, Ozdamar O. Objective analysis of early auditory responses elicited by gaps in noise. Ppapr presented at: SoutheastCon. 09-12 April 2015; Fort Lauderdale, FL, USA. [DOI:10.1109/SECON.2015.7132953]
Type of Study: Review Article |
Subject:
Audiology
Received: 2023/07/13 | Accepted: 2023/08/9 | Published: 2023/02/4
Received: 2023/07/13 | Accepted: 2023/08/9 | Published: 2023/02/4








