Volume 5, Issue 1 (Continuously Updated 2022)
Func Disabil J 2022, 5(1): 0-0 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hosseini S M, Mazaher Yazdi M, Maarefvand M, Haddadzadehniri H, Heidari A. Comparison of the Components of Brainstem Auditory Responses Using Click and Chirp Stimuli in Premature Infants. Func Disabil J 2022; 5 (1) : 66
URL: http://fdj.iums.ac.ir/article-1-211-en.html
URL: http://fdj.iums.ac.ir/article-1-211-en.html
Seyedeh Maryam Hosseini1 

 , Maliheh Mazaher Yazdi2
, Maliheh Mazaher Yazdi2 

 , Mohammad Maarefvand *2
, Mohammad Maarefvand *2 

 , Hasan Haddadzadehniri2
, Hasan Haddadzadehniri2 

 , Atta Heidari3
, Atta Heidari3 




 , Maliheh Mazaher Yazdi2
, Maliheh Mazaher Yazdi2 

 , Mohammad Maarefvand *2
, Mohammad Maarefvand *2 

 , Hasan Haddadzadehniri2
, Hasan Haddadzadehniri2 

 , Atta Heidari3
, Atta Heidari3 


1- Department of Audiology, School of Rehabilitation Sciences, Rehabilitation Research Center, Iran University of Medical Sciences, Tehran, Iran
2- Department of Audiology, School of Rehabilitation Sciences, Rehabilitation Research Center, Iran University of Medical Sciences, Tehran, Iran.
3- Department of Audiology, School of Rehabilitation Sciences, Rehabilitation Research Center, Hamedan University of Medical Sciences, Hamedan, Iran.
2- Department of Audiology, School of Rehabilitation Sciences, Rehabilitation Research Center, Iran University of Medical Sciences, Tehran, Iran.
3- Department of Audiology, School of Rehabilitation Sciences, Rehabilitation Research Center, Hamedan University of Medical Sciences, Hamedan, Iran.
Full-Text [PDF 1103 kb]
(431 Downloads)
| Abstract (HTML) (1335 Views)
Full-Text: (463 Views)
Introduction
According to the definition by the World Health Organization (WHO), babies who are born before 37 weeks of pregnancy or less than 259 days from the first day of the mother’s last period are called premature infants [1] The probability of hearing disorders in premature infants with low gestational age is higher than in mature infants, and the history of hospitalization in the Neonatal intensive care unit (NICU) after this premature birth also increases this probability [2-4]. Long-term use of breathing tubes makes these infants susceptible to middle ear infections. Low birth weight, environmental noises caused by the incubator used due to the Hyperbilirubinemia, as well as prescribed antibiotics can be complications of premature birth that may lead to hearing problems in this group of infants [3, 4]. One of the most important causes of hearing problems in these infants is cerebral white matter injury, which disrupts the normal development of the myelination of auditory nerve pathways and affects the normal maturation of the auditory system. Myelination and maturation of the auditory nervous system occur from the peripheral part to the central part [5].
If hearing loss is diagnosed before one month of age and treatment measures are taken before three months of age, the prognosis will be very good; thus, early diagnosis of hearing disorders at a young age prevents disabilities caused by late diagnosis of hearing disorders [6, 7]. Considering the maturation process and many differences in the auditory evoked brainstem response (ABR) components of premature and mature infants, using the values of ABR components related to premature infants to prevent diagnostic errors, more accurate and faster assessment of retro-cochlear injuries seems necessary in this group of infants [8, 9]. Click stimulus is used to estimate the hearing threshold in infants, but this stimulus is only able to estimate the hearing sensitivity in the high-frequency areas of the cochlea [10, 11]. Chirp stimulus is designed to compensate for cochlear delay and firing asynchrony of cochlear basal and apex nerve units, which can increase the synchronization between nerve units in different cochlear regions and lead to larger amplitudes. In this case, stronger responses are expected [10, 12, 13]. The purpose of this study was to investigate the response of the auditory brainstem to the click and chirp stimulus in premature and mature infants.
Materials and Methods
Four groups of 20 infants (a total of 80 infants) consisting of three groups of premature and one group of mature infants participated in this study, and the division of premature infants according to the gestational age of the mother at the time of birth was according to Table 1 [14]. It should be mentioned in tables paired t-test was used in cases marked with *. Wilcoxon test was used in other cases.

The studied population was newborns who were referred to Fatemiyeh Hospital in Hamadan. Assessments were conducted in a relatively quiet room in the intensive care unit. The necessary information in this research was collected through interviews, examination, and clinical evaluation. Entry criteria for preterm infants included an age range from 28 weeks to before 37 weeks of pregnancy. Term infants were at 37-40 weeks of gestation to enter the intervention. Other inclusion criteria for both term and preterm groups were the same and included the absence of hearing problems in family members, the absence of genetic and syndromic sensory and motor diseases diagnosed by a pediatrician, confirmation of hearing health using a set of tests, including otoscopy, tympanometry, transitory evoked otoacoustic emission (TEOAE), behavioral observation audiometry (BOA), and non-use of ototoxic drugs by the mother during pregnancy. Parents’ unwillingness to continue the research process or observe any abnormality in the ABR test, such as neuropathy disorder was one of the exclusion criteria. In case of lack of auditory evoked response, weak morphology of ABR waves, and suspicion of hearing loss, the infant was excluded from the test process and referred for further evaluation.
All infants were evaluated by click and chirp stimuli. The components examined in the ABR test included the amplitude and latency of waves I, III, and V, interpeak intervals of I-III, III-V, and I-V, as well as the V/I amplitude ratio. The secondary evaluation was also performed approximately three months later under the same conditions. Stimulus intensity was 80 and 40 dB to compare the difference in response to stimuli at high- and near-threshold intensity levels. Recording the evoked responses of the baby in sleep mode and in a quiet environment using expansion click and 1.0-ms diversion and chirp stimulus with a wide frequency range [13], using the inserted phone as a single phone and with 2000 number of sweeps and the stimulus rate was 27.3C⁄S with a bandpass filter of 100-3000 Hz. The time window was set at 15 ms with a pre-stimulation time of -1 ms and 100,000 times amplification. ABR waves were recorded through electrodes on the forehead, bilateral mastoid, and vertex [15].
The comparison of click and chirp stimuli, inter-group comparisons, and analysis of response changes over several months were done by the paired t-test, Wilcoxon test, and Kruskal-Wallis test and using SPSS software, version 18.
Results
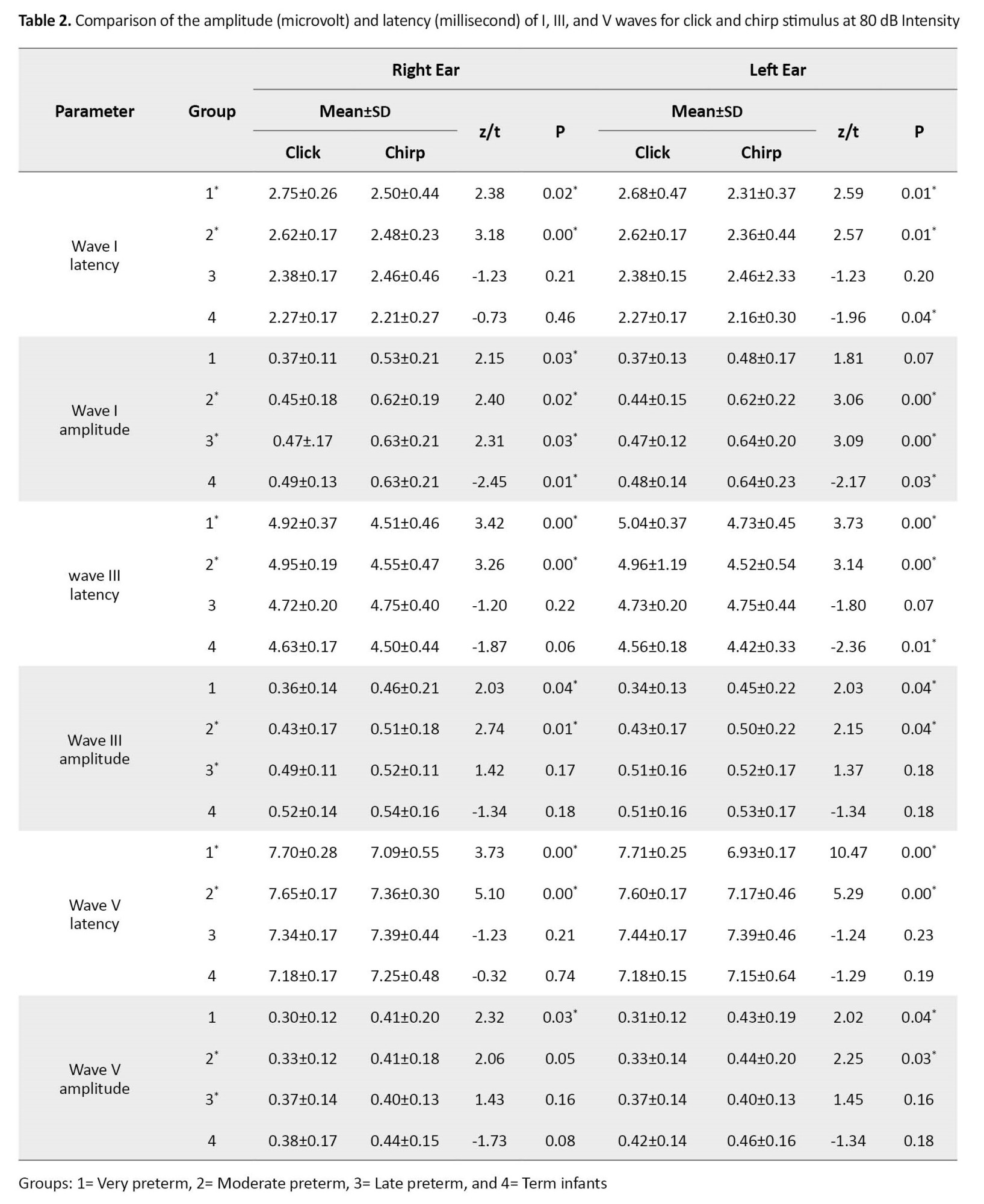
The comparison of the results obtained from the click and chirp stimuli separately in each group at 80 dB intensity is shown in Table 2. No significant difference was found between the interpeak latency and V/I ratio of stimuli (p˃0.05); thus, they were omitted. The amplitude and latency of waves I, III, and V mainly showed a significant difference between the click and chirp stimuli so that the chirp stimulus had a shorter latency and a larger response amplitude at the intensity of 80 dB (p˂0.05). For example, in the first row of the table indicating data about the first group of very preterm infants in the right ear, the latency of wave I for the chirp stimulus was equal to 2.50±0.44, while this value was 2.75±0.26 for the click stimulus. These values for chirp wave III and V latency were equal to 4.51±0.46 and 7.09±0.55, which were higher for the click stimulus (4.92±0.37 and 7.70±0.28, respectively). The same findings were also obtained regarding the wave latency in other groups. The findings of latency and amplitude of waves indicated that the chirp stimulus in premature infants can be a better stimulus than the conventional click stimulus for recording responses.
In the second step, a between-group comparison of the data of all four groups was done using the Kruskal-Wallis non-parametric test (Table 3). There was a significant difference between the latency of waves I, III, and V in these groups (p˂0.05), but this finding was not obtained regarding the amplitude of all waves (p˃0.05). The parameter of interpeak latency and V/I ratio showed no significant difference (p˃0.05).
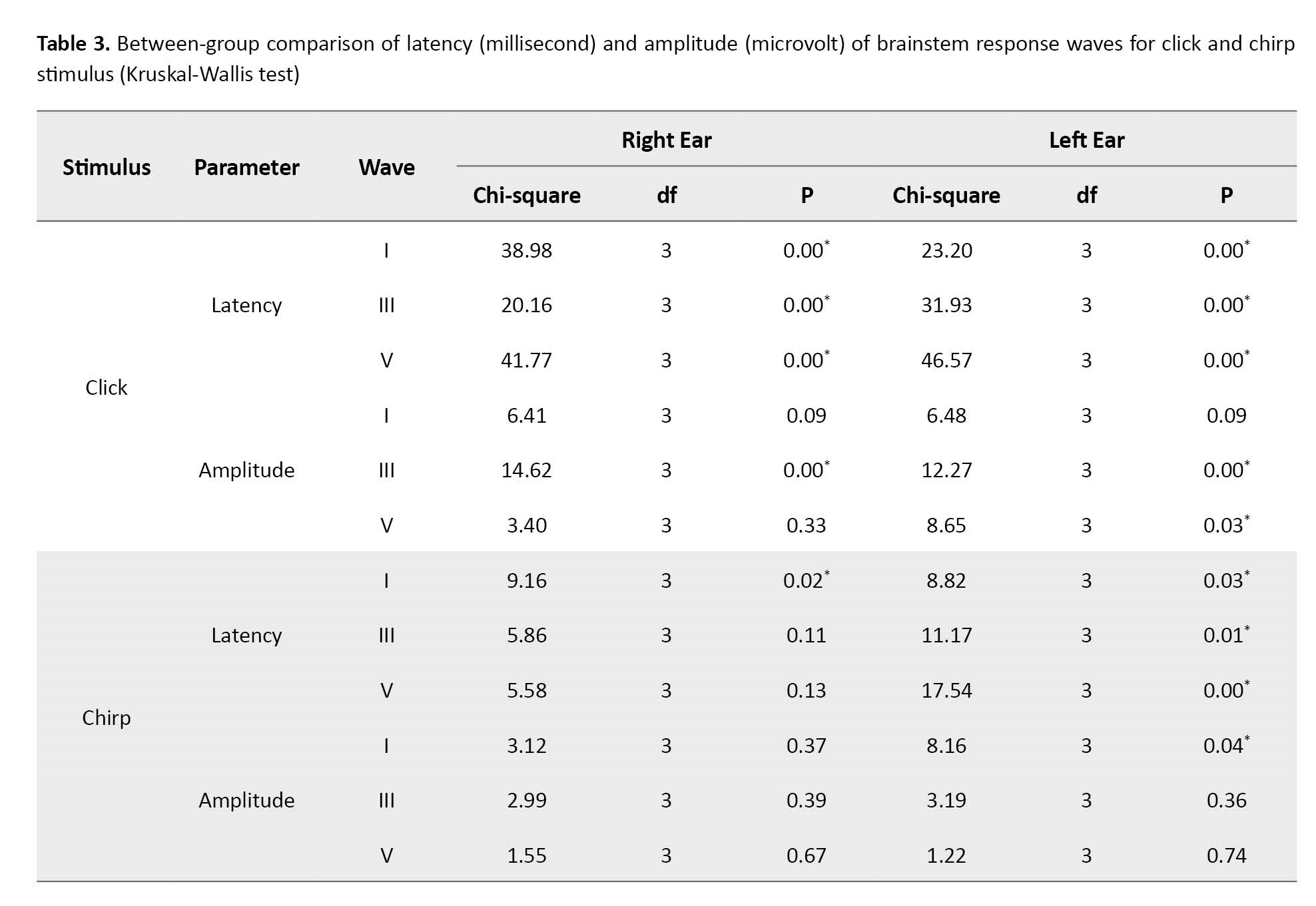
The chirp stimulus at the intensity of 40 dB mainly had a longer latency and a lower amplitude than the click stimulus only in groups 3 and 4 (p˂0.05). In groups 1 and 2, which included more premature infants than other groups, the chirp stimulus latency did not show a significant difference compared to the click stimulus (p˃0.05). For brevity, these findings are not mentioned in this section.
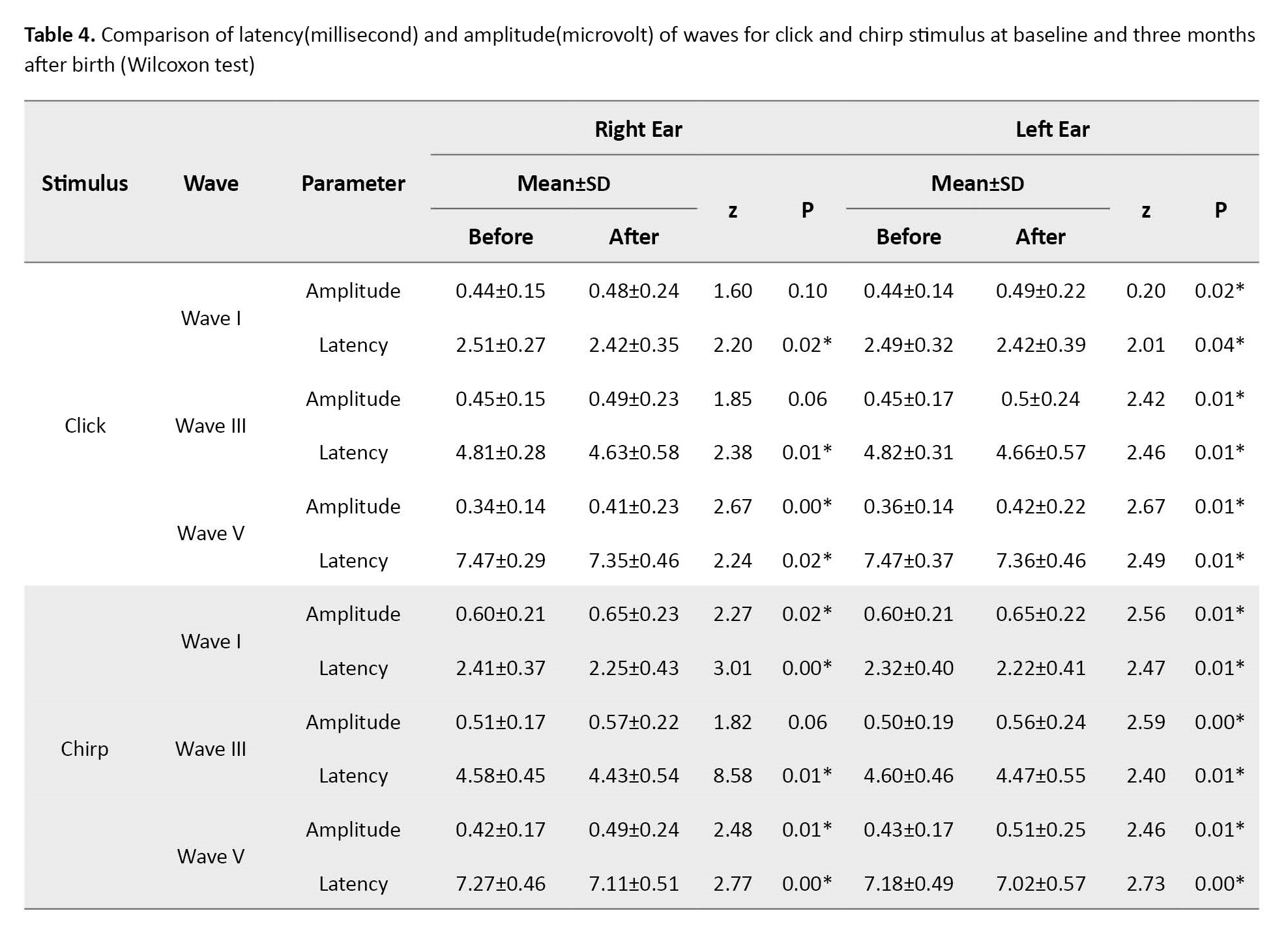
Re-evaluation three months after birth in all groups showed an increase in amplitude and a decrease in the latency of the waves (p˂0.05), which can be seen as a sign of the continuation of the evolution of responses after birth (Table 4).
Discussion
The efficiency of chirp stimulus at high-intensity levels (80 dB)
Brainstem-evoked responses of infants for two stimuli, click and chirp, were investigated in the present study. The main difference between our study and previous studies was that they mainly evaluated normal adults or term infants, while we attempted to investigate the effectiveness of the chirp stimulus in different groups of preterm infants with different gestational ages to understand whether this stimulus used for all these premature infants is effective or not. Previous studies have shown that the click and chirp stimuli have differences in creating excited responses in normal adults and the chirp stimulus can be more useful [16, 17]. For example, Sabet et al. in 2014 assessed the differences between click and chirp stimuli and showed that the chirp stimulus is capable of producing evoked responses with less latency than the click stimulus at an intensity of 80 dB [11]. They studied normal-hearing adults. Research has generally shown that the click stimulus has less power in creating a nervous response [18-20]. The chirp stimulus can reduce intra-cochlear temporal dispersions and enable the recording of neural responses with higher amplitude and shorter acquisition time [13, 21, 22]. In 2021, Ceylan et al. compared brainstem waves induced by click and chirp stimuli in patients with unilateral hearing loss [23]. Studies have also been conducted in the field of infants, but they have used term infants; for example, da Silva Ormundo and Lewis in 2021 used click and chirp stimuli in term infants with normal hearing to record brainstem auditory evoked responses [21]. Responses were recorded at an intensity of 70 dB. The use of chirp stimulus for term infants as a more suitable stimulus for extracting waves with better morphology and higher V wave amplitude was one of the results of this study.
The chirp stimulus in the present study mainly produced a stronger auditory evoked brainstem response in term and premature infants (Table 1). The lower latency time and the larger response amplitude for the chirp stimulus can be seen as a sign of greater neural synchrony in generating such responses.
Effect of gestational age on brainstem evoked responses
In the present study, the gestational age of the mother at the time of birth was an effective factor regarding how the auditory system responds to different stimuli. There was a significant difference, especially in the latency parameter of all three waves, between the different groups (Table 2). Mohammadkhani et al. in 2009 [8] showed a significant difference between the brainstem-evoked responses of normal and premature infants. They only used the click stimulus. They attributed this difference to the immaturity of the auditory system in premature infants. However, there are also studies showing that the latency of wave I is not affected by the prematurity of infants, and the process of maturation of the auditory pathways of premature infants is the same. Therefore, prematurity is a risk factor that cannot alone affect the evolution process of the brainstem response [24, 25]. This difference between their findings and the present study may be related to the small sample size in both studies. In general, the older the mother’s gestational age at the time of the birth of the infants, the shorter the latency of the response, and in some cases, they were recorded with a higher amplitude. These findings can be seen as a sign of a delay in the maturation of the system and myelination of the central pathways and a kind of non-synchronicity in the responses, which has been seen due to the prematurity of the infants, which is confirmed by previous studies [8, 25, 26]. With age, the responses become stronger.
Improving responses in secondary assessment
In the present study, with the passage of time from the initial assessment in all groups, the improvement of responses was observed (Table 3) so that the amplitudes increased and the absolute latency of the waves decreased, which was also consistent with previous studies [27]. These differences show that it is better to use the norms of this group to diagnose retro-cochlear injuries and even threshold evaluation in premature infants. In 2019, Seethapathy et al. investigated brainstem evoked response in 80 very preterm and late preterm infants at one and three months of age [25]. The click stimulus was used at the intensity of 70 and 30 dB nHL. This study showed that the maturation process of the auditory system is from the periphery to the center and does not differ in different groups of premature infants. Infants who were very premature had less neuronal development than the other group, which accelerated with age. These findings can confirm the results of the present study.
Behavior of the chirp stimulus at low-intensity levels
In this research, as can be seen at high-intensity levels, such as 80 dB, the chirp response had lower latency than the click stimulus, but at the intensity of 40 dB, the behavior of this stimulus was different. There were differences in the response to the chirp stimulus in the different groups. In groups 3 and 4, whose infants were more developed, the latency of the chirp stimulus was higher than that of the click stimulus, which is in line with Khorsand’s findings that 20-30-year-old normal adults had examined. Sabet et al. showed that the chirp stimulus at low-intensity levels, i.e. 20 and 40 dB, had a higher latency than the click stimulus [11]. This difference in response to the chirp stimulus has also been reported in other studies (for example, the study by Ceylan on patients with bilateral hearing loss [23]). However, our findings regarding more premature infants, namely groups 1 and 2, are different from the findings of other groups (3 and 4) because, in these groups, no significant difference was observed between V wave latency for chirp and click stimuli. Because there is no similar finding in preterm infants in previous studies to compare this result, it can be said that the chirp stimulus can be an effective stimulus at low- and high-intensity levels in preterm infants because there is no more latency problem for the chirp, at least in these premature infants.
Conclusion
Greater amplitude and lower latency than the click stimulus were created by the chirp stimulus in the present study. The process of estimating thresholds in preterm infants can be difficult due to their less development than term infants; thus, a better stimulus, such as a chirp, will be able to elicit stronger responses and make this process easier because it is closer to the behavioral threshold and the hearing threshold is achieved in a shorter time.
It is recommended to use this stimulus in other groups of patients with hearing disorders and evaluate it in groups with a higher number of samples to determine the norm values of this test in future studies. On the other hand, we hope that this research can pave the way for widespread clinical use of this stimulus for better diagnosis of hearing loss in infants so that evaluation protocols can be improved.
Ethical Considerations
Compliance with ethical guidelines
The code of ethics was received from the Ethics Committee of the Iran University of Medical Sciences (IR.IUMS.REC.1401.352).
Funding
The study was extracted from the MSc thesis entitled "Comparison of components of brainstem auditory responses using click and chirp stimuli in premature infants" in 2023 by Seyedeh Maryam Hosseini approved and funded by Iran university of medical Sciences.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
According to the definition by the World Health Organization (WHO), babies who are born before 37 weeks of pregnancy or less than 259 days from the first day of the mother’s last period are called premature infants [1] The probability of hearing disorders in premature infants with low gestational age is higher than in mature infants, and the history of hospitalization in the Neonatal intensive care unit (NICU) after this premature birth also increases this probability [2-4]. Long-term use of breathing tubes makes these infants susceptible to middle ear infections. Low birth weight, environmental noises caused by the incubator used due to the Hyperbilirubinemia, as well as prescribed antibiotics can be complications of premature birth that may lead to hearing problems in this group of infants [3, 4]. One of the most important causes of hearing problems in these infants is cerebral white matter injury, which disrupts the normal development of the myelination of auditory nerve pathways and affects the normal maturation of the auditory system. Myelination and maturation of the auditory nervous system occur from the peripheral part to the central part [5].
If hearing loss is diagnosed before one month of age and treatment measures are taken before three months of age, the prognosis will be very good; thus, early diagnosis of hearing disorders at a young age prevents disabilities caused by late diagnosis of hearing disorders [6, 7]. Considering the maturation process and many differences in the auditory evoked brainstem response (ABR) components of premature and mature infants, using the values of ABR components related to premature infants to prevent diagnostic errors, more accurate and faster assessment of retro-cochlear injuries seems necessary in this group of infants [8, 9]. Click stimulus is used to estimate the hearing threshold in infants, but this stimulus is only able to estimate the hearing sensitivity in the high-frequency areas of the cochlea [10, 11]. Chirp stimulus is designed to compensate for cochlear delay and firing asynchrony of cochlear basal and apex nerve units, which can increase the synchronization between nerve units in different cochlear regions and lead to larger amplitudes. In this case, stronger responses are expected [10, 12, 13]. The purpose of this study was to investigate the response of the auditory brainstem to the click and chirp stimulus in premature and mature infants.
Materials and Methods
Four groups of 20 infants (a total of 80 infants) consisting of three groups of premature and one group of mature infants participated in this study, and the division of premature infants according to the gestational age of the mother at the time of birth was according to Table 1 [14]. It should be mentioned in tables paired t-test was used in cases marked with *. Wilcoxon test was used in other cases.

The studied population was newborns who were referred to Fatemiyeh Hospital in Hamadan. Assessments were conducted in a relatively quiet room in the intensive care unit. The necessary information in this research was collected through interviews, examination, and clinical evaluation. Entry criteria for preterm infants included an age range from 28 weeks to before 37 weeks of pregnancy. Term infants were at 37-40 weeks of gestation to enter the intervention. Other inclusion criteria for both term and preterm groups were the same and included the absence of hearing problems in family members, the absence of genetic and syndromic sensory and motor diseases diagnosed by a pediatrician, confirmation of hearing health using a set of tests, including otoscopy, tympanometry, transitory evoked otoacoustic emission (TEOAE), behavioral observation audiometry (BOA), and non-use of ototoxic drugs by the mother during pregnancy. Parents’ unwillingness to continue the research process or observe any abnormality in the ABR test, such as neuropathy disorder was one of the exclusion criteria. In case of lack of auditory evoked response, weak morphology of ABR waves, and suspicion of hearing loss, the infant was excluded from the test process and referred for further evaluation.
All infants were evaluated by click and chirp stimuli. The components examined in the ABR test included the amplitude and latency of waves I, III, and V, interpeak intervals of I-III, III-V, and I-V, as well as the V/I amplitude ratio. The secondary evaluation was also performed approximately three months later under the same conditions. Stimulus intensity was 80 and 40 dB to compare the difference in response to stimuli at high- and near-threshold intensity levels. Recording the evoked responses of the baby in sleep mode and in a quiet environment using expansion click and 1.0-ms diversion and chirp stimulus with a wide frequency range [13], using the inserted phone as a single phone and with 2000 number of sweeps and the stimulus rate was 27.3C⁄S with a bandpass filter of 100-3000 Hz. The time window was set at 15 ms with a pre-stimulation time of -1 ms and 100,000 times amplification. ABR waves were recorded through electrodes on the forehead, bilateral mastoid, and vertex [15].
The comparison of click and chirp stimuli, inter-group comparisons, and analysis of response changes over several months were done by the paired t-test, Wilcoxon test, and Kruskal-Wallis test and using SPSS software, version 18.
Results
The comparison of the results obtained from the click and chirp stimuli separately in each group at 80 dB intensity is shown in Table 2. No significant difference was found between the interpeak latency and V/I ratio of stimuli (p˃0.05); thus, they were omitted. The amplitude and latency of waves I, III, and V mainly showed a significant difference between the click and chirp stimuli so that the chirp stimulus had a shorter latency and a larger response amplitude at the intensity of 80 dB (p˂0.05). For example, in the first row of the table indicating data about the first group of very preterm infants in the right ear, the latency of wave I for the chirp stimulus was equal to 2.50±0.44, while this value was 2.75±0.26 for the click stimulus. These values for chirp wave III and V latency were equal to 4.51±0.46 and 7.09±0.55, which were higher for the click stimulus (4.92±0.37 and 7.70±0.28, respectively). The same findings were also obtained regarding the wave latency in other groups. The findings of latency and amplitude of waves indicated that the chirp stimulus in premature infants can be a better stimulus than the conventional click stimulus for recording responses.

In the second step, a between-group comparison of the data of all four groups was done using the Kruskal-Wallis non-parametric test (Table 3). There was a significant difference between the latency of waves I, III, and V in these groups (p˂0.05), but this finding was not obtained regarding the amplitude of all waves (p˃0.05). The parameter of interpeak latency and V/I ratio showed no significant difference (p˃0.05).

The chirp stimulus at the intensity of 40 dB mainly had a longer latency and a lower amplitude than the click stimulus only in groups 3 and 4 (p˂0.05). In groups 1 and 2, which included more premature infants than other groups, the chirp stimulus latency did not show a significant difference compared to the click stimulus (p˃0.05). For brevity, these findings are not mentioned in this section.
Re-evaluation three months after birth in all groups showed an increase in amplitude and a decrease in the latency of the waves (p˂0.05), which can be seen as a sign of the continuation of the evolution of responses after birth (Table 4).

Discussion
The efficiency of chirp stimulus at high-intensity levels (80 dB)
Brainstem-evoked responses of infants for two stimuli, click and chirp, were investigated in the present study. The main difference between our study and previous studies was that they mainly evaluated normal adults or term infants, while we attempted to investigate the effectiveness of the chirp stimulus in different groups of preterm infants with different gestational ages to understand whether this stimulus used for all these premature infants is effective or not. Previous studies have shown that the click and chirp stimuli have differences in creating excited responses in normal adults and the chirp stimulus can be more useful [16, 17]. For example, Sabet et al. in 2014 assessed the differences between click and chirp stimuli and showed that the chirp stimulus is capable of producing evoked responses with less latency than the click stimulus at an intensity of 80 dB [11]. They studied normal-hearing adults. Research has generally shown that the click stimulus has less power in creating a nervous response [18-20]. The chirp stimulus can reduce intra-cochlear temporal dispersions and enable the recording of neural responses with higher amplitude and shorter acquisition time [13, 21, 22]. In 2021, Ceylan et al. compared brainstem waves induced by click and chirp stimuli in patients with unilateral hearing loss [23]. Studies have also been conducted in the field of infants, but they have used term infants; for example, da Silva Ormundo and Lewis in 2021 used click and chirp stimuli in term infants with normal hearing to record brainstem auditory evoked responses [21]. Responses were recorded at an intensity of 70 dB. The use of chirp stimulus for term infants as a more suitable stimulus for extracting waves with better morphology and higher V wave amplitude was one of the results of this study.
The chirp stimulus in the present study mainly produced a stronger auditory evoked brainstem response in term and premature infants (Table 1). The lower latency time and the larger response amplitude for the chirp stimulus can be seen as a sign of greater neural synchrony in generating such responses.
Effect of gestational age on brainstem evoked responses
In the present study, the gestational age of the mother at the time of birth was an effective factor regarding how the auditory system responds to different stimuli. There was a significant difference, especially in the latency parameter of all three waves, between the different groups (Table 2). Mohammadkhani et al. in 2009 [8] showed a significant difference between the brainstem-evoked responses of normal and premature infants. They only used the click stimulus. They attributed this difference to the immaturity of the auditory system in premature infants. However, there are also studies showing that the latency of wave I is not affected by the prematurity of infants, and the process of maturation of the auditory pathways of premature infants is the same. Therefore, prematurity is a risk factor that cannot alone affect the evolution process of the brainstem response [24, 25]. This difference between their findings and the present study may be related to the small sample size in both studies. In general, the older the mother’s gestational age at the time of the birth of the infants, the shorter the latency of the response, and in some cases, they were recorded with a higher amplitude. These findings can be seen as a sign of a delay in the maturation of the system and myelination of the central pathways and a kind of non-synchronicity in the responses, which has been seen due to the prematurity of the infants, which is confirmed by previous studies [8, 25, 26]. With age, the responses become stronger.
Improving responses in secondary assessment
In the present study, with the passage of time from the initial assessment in all groups, the improvement of responses was observed (Table 3) so that the amplitudes increased and the absolute latency of the waves decreased, which was also consistent with previous studies [27]. These differences show that it is better to use the norms of this group to diagnose retro-cochlear injuries and even threshold evaluation in premature infants. In 2019, Seethapathy et al. investigated brainstem evoked response in 80 very preterm and late preterm infants at one and three months of age [25]. The click stimulus was used at the intensity of 70 and 30 dB nHL. This study showed that the maturation process of the auditory system is from the periphery to the center and does not differ in different groups of premature infants. Infants who were very premature had less neuronal development than the other group, which accelerated with age. These findings can confirm the results of the present study.
Behavior of the chirp stimulus at low-intensity levels
In this research, as can be seen at high-intensity levels, such as 80 dB, the chirp response had lower latency than the click stimulus, but at the intensity of 40 dB, the behavior of this stimulus was different. There were differences in the response to the chirp stimulus in the different groups. In groups 3 and 4, whose infants were more developed, the latency of the chirp stimulus was higher than that of the click stimulus, which is in line with Khorsand’s findings that 20-30-year-old normal adults had examined. Sabet et al. showed that the chirp stimulus at low-intensity levels, i.e. 20 and 40 dB, had a higher latency than the click stimulus [11]. This difference in response to the chirp stimulus has also been reported in other studies (for example, the study by Ceylan on patients with bilateral hearing loss [23]). However, our findings regarding more premature infants, namely groups 1 and 2, are different from the findings of other groups (3 and 4) because, in these groups, no significant difference was observed between V wave latency for chirp and click stimuli. Because there is no similar finding in preterm infants in previous studies to compare this result, it can be said that the chirp stimulus can be an effective stimulus at low- and high-intensity levels in preterm infants because there is no more latency problem for the chirp, at least in these premature infants.
Conclusion
Greater amplitude and lower latency than the click stimulus were created by the chirp stimulus in the present study. The process of estimating thresholds in preterm infants can be difficult due to their less development than term infants; thus, a better stimulus, such as a chirp, will be able to elicit stronger responses and make this process easier because it is closer to the behavioral threshold and the hearing threshold is achieved in a shorter time.
It is recommended to use this stimulus in other groups of patients with hearing disorders and evaluate it in groups with a higher number of samples to determine the norm values of this test in future studies. On the other hand, we hope that this research can pave the way for widespread clinical use of this stimulus for better diagnosis of hearing loss in infants so that evaluation protocols can be improved.
Ethical Considerations
Compliance with ethical guidelines
The code of ethics was received from the Ethics Committee of the Iran University of Medical Sciences (IR.IUMS.REC.1401.352).
Funding
The study was extracted from the MSc thesis entitled "Comparison of components of brainstem auditory responses using click and chirp stimuli in premature infants" in 2023 by Seyedeh Maryam Hosseini approved and funded by Iran university of medical Sciences.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
- Rao CR, de Ruiter LE, Bhat P, Kamath V, Kamath A, Bhat V. A case-control study on risk factors for preterm deliveries in a secondary care hospital, southern India. ISRN Obstet Gynecol. 2014; 2014:935982. [DOI:10.1155/2014/935982] [PMID] [PMCID]
- Borradori C, Fawer CL, Buclin T, Calame A. Risk factors of sensorineural hearing loss in preterm infants. Biol Neonate. 1997; 71(1):1-10. [DOI:10.1159/000244391] [PMID]
- Casali RL, Santos MF. Auditory Brainstem Evoked Response: Response patterns of full-term and premature infants. Braz J Otorhinolaryngol. 2010; 76(6):729-38. [DOI:10.1590/S1808-86942010000600011] [PMID]
- Frezza S, Catenazzi P, Gallus R, Gallini F, Fioretti M, Anzivino R, et al. Hearing loss in very preterm infants: Should we wait or treat? Acta Otorhinolaryngol Ital. 2019; 39(4):257–62. [DOI:10.14639/0392-100X-2116] [PMID] [PMCID]
- Back SA. White matter injury in the preterm infant: Pathology and mechanisms. Acta Neuropathol. 2017; 134(3):331-49. [DOI:10.1007/s00401-017-1718-6] [PMID] [PMCID]
- Mazaheryazdi M, Akbari M, Abolhasan Choobdar F. Auditory and speech development in a 3-year-old boy with auditory neuropathy spectrum disorder. Aud Vestib Res. 2021; 30(2):139-47. [DOI:10.18502/avr.v30i2.6099]
- Olusanya BO. Highlights of the new WHO Report on Newborn and Infant Hearing Screening and implications for developing countries. Int J Pediatr Otorhinolaryngol. 2011; 75(6):745-8. [DOI:10.1016/j.ijporl.2011.01.036] [PMID]
- Mohammadkhani G, Haji Ebrahim Tehrani F, Sheykhzadeh M, Majidi H, Faghihzadeh S. [Comparing the latency and amplitude of auditory-evoked brainstem responses in full-term and premature neonates (Persian)]. Feyz. 2009; 13(2):90-3. [Link]
- Ness DA. Normative data for neurodiagnostic Auditory Brainstem Response testing (ABR) [PhD thesis]. Louisiana: Louisiana Tech University; 2009. [Link]
- Hall JW. New handbook of auditory evoked responses. London: Pearson; 2007. [Link]
- Khorsand Sabet V, Mahdavi-Zafarghandi ME, Safavi M, Sharifian M, Tabatabaee SM. [Comparison of click and CE-chirp-evoked human auditory brainstem responses: A preliminary study (Persian)]. Audiology. 2014; 23(4):69-76. [Link]
- Elberling C, Callø J, Don M. Evaluating auditory brainstem responses to different chirp stimuli at three levels of stimulation. J Acoust Soc Am. 2010; 128(1):215-23. [DOI:10.1121/1.3397640] [PMID] [PMCID]
- Elberling C, Don M. Auditory brainstem responses to a chirp stimulus designed from derived-band latencies in normal-hearing subjects. J Acoust Soc Am. 2008; 124(5):3022-37. [DOI:10.1121/1.2990709] [PMID] [PMCID]
- No Author. Neonatal-perinatal medicine: Diseases of the fetus and infant. Arch Dis Child. 1987; 53(8):696. [DOI:10.1136/adc.53.8.696-e] [PMID]
- Hall JW, Swanepoel DW. Objective assessment of hearing. San Diego: Plural Publishing; 2009. [Link]
- Pettigrew CM, Murdoch BE, Ponton CW, Kei J, Chenery HJ, Alku P. Subtitled videos and mismatch negativity (MMN) investigations of spoken word processing. J Am Acad Audiol. 2004; 15(07):469-85. [DOI:10.3766/jaaa.15.7.2] [PMID]
- Sena TA, Ramos N, Rodrigues GR, Lewis DR. Testing time comparison between two procedures with new technologies of Automated Auditory Brainstem Response (AABR). Codas. 2013; 25(1):34-8. [DOI:10.1590/S2317-17822013000100007] [PMID]
- Dau T, Wegner O, Mellert V, Kollmeier B. Auditory brainstem responses with optimized chirp signals compensating basilar-membrane dispersion. J Acoust Soc Am. 2000; 107(3):1530-40. [DOI:10.1121/1.428438] [PMID]
- Fobel O, Dau T. Searching for the optimal stimulus eliciting auditory brainstem responses in humans. J Acoust Soc Am. 2004; 116(4):2213-22. [DOI:10.1121/1.1787523] [PMID]
- Shore SE, Nuttall AL. High-synchrony cochlear compound action potentials evoked by rising frequency-swept tone bursts. J Acoust Soc Am. 1985; 78(4):1286-95. [DOI:10.1121/1.392898] [PMID]
- Ormundo DDS, Lewis DR. Auditory brainstem response with click and CE-Chirp® Level Specific stimuli in hearing infants. Int J Pediatr Otorhinolaryngol. 2021; 147:110819. [DOI:10.1016/j.ijporl.2021.110819] [PMID]
- Elberling C, Don M, Cebulla M, Stürzebecher E. Auditory steady-state responses to chirp stimuli based on cochlear traveling wave delay. J Acoust Soc Am. 2007; 122(5):2772-85. [DOI:10.1121/1.2783985] [PMID]
- Ceylan S, Gümüşgün A, Feratlar F. Comparison of CE-Chirp ABR and Click ABR methods in patients with bilateral sensorineural hearing loss. ENT Updates. 2018; 8(1):27-32. [DOI:10.2399/jmu.2018001009]
- Seethapathy J, Boominathan P, Uppunda AK, Ninan B. Auditory brainstem response in very preterm, moderately preterm and late preterm infants. Int J Pediatr Otorhinolaryngol. 2018; 111:119-27. [DOI:10.1016/j.ijporl.2018.06.006] [PMID]
- Seethapathy J, Boominathan P, Uppunda AK, Ninan B. Changes in auditory brainstem response in very preterm and late preterm infants. Int J Pediatr Otorhinolaryngol. 2019; 121:88-94. [DOI:10.1016/j.ijporl.2019.03.008] [PMID]
- Starr A, Amlie RN, Martin WH, Sanders S. Development of auditory function in newborn infants revealed by auditory brainstem potentials. Pediatrics. 1977; 60(6):831-9. [DOI:10.1542/peds.60.6.831] [PMID]
- Cox C, Hack M, Metz D. Brainstem-evoked response audiometry: Normative data from the preterm infant. Audiology. 1981; 20(1):53-64. [DOI:10.3109/00206098109072682] [PMID]
Type of Study: Research |
Subject:
Audiology
Received: 2023/02/13 | Accepted: 2023/04/18 | Published: 2022/02/3
Received: 2023/02/13 | Accepted: 2023/04/18 | Published: 2022/02/3





