Volume 5, Issue 1 (Continuously Updated 2022)
Func Disabil J 2022, 5(1): 0-0 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Qodrati Tavana A, Mirsaeedi A S, Ghasisin L, Bemani Z. Second Formant Transition Characteristics in Persian-Speaking People With Broca’s Aphasia. Func Disabil J 2022; 5 (1) : 76
URL: http://fdj.iums.ac.ir/article-1-197-en.html
URL: http://fdj.iums.ac.ir/article-1-197-en.html
1- Department of English Language, School of Management and Medical Information, Isfahan University of Medical Sciences, Isfahan, Iran.
2- Department of English Language, Faculty of Foreign Languages, Isfahan (Khorasgan) Branch, Islamic Azad University, Isfahan, Iran.
3- Department of Speech Therapy, Communication Disorders Research Center, School of Rehabilitation Sciences, Isfahan University of Medical Sciences, Isfahan, Iran.
4- Department of Speech Therapy, Rehabilitation Research Center, School of Rehabilitation Sciences, Iran University of Medical Sciences, Tehran, Iran. ,zahrabemani60@yahoo.com
2- Department of English Language, Faculty of Foreign Languages, Isfahan (Khorasgan) Branch, Islamic Azad University, Isfahan, Iran.
3- Department of Speech Therapy, Communication Disorders Research Center, School of Rehabilitation Sciences, Isfahan University of Medical Sciences, Isfahan, Iran.
4- Department of Speech Therapy, Rehabilitation Research Center, School of Rehabilitation Sciences, Iran University of Medical Sciences, Tehran, Iran. ,
Full-Text [PDF 1183 kb]
(361 Downloads)
| Abstract (HTML) (1110 Views)
Full-Text: (483 Views)
Introduction
Aphasia is an acquired language communication disorder caused by stroke, tumor, or brain injury characterized by a disorder in language skills, such as speaking, comprehension, reading, and writing. Different types of aphasia with diverse clinical features exist. Broca’s aphasia is one of the most familiar and widespread types of aphasia, classified as non-fluent aphasia. The prominent features of speech in people with Broca’s aphasia include limited and non-fluent verbal output produced with many efforts and pauses, reduced speech rate, reduced speech length, inaccurate production of sounds, agrammatism, and impaired speech tone [1]. One of the clinical manifestations of Broca’s aphasia is a deficit in speech articulation. Various studies have shown that patients with Broca’s aphasia suffer from deficits that affect the structure of sounds in their speech output. Determining the nature of speech errors in these patients is strictly necessary and crucial in diagnosing the disturbed and effective mechanisms that cause these errors [2].
On the other hand, the production of speech sounds requires the integration of auditory, somatosensory, and motor information represented in the temporal, parietal, and frontal lobes. In addition to subcortical structures, cortical areas, and their functional connections form a functional unit called the speech-motor control system [3]. Speech motor control refers to systems and strategies which control speech production. Some sets of productive movements convert the desired language message into an acoustic signal interpreted by the listener, including the input of the speech motor control system, linguistic representations, especially the sequence of abstract units, i.e. phonemes, and also the output of the speech motor control system. Therefore, the speech motor control system is located between the two processes of language formulation and the creation of an acoustic signal that transmits the message [4, 5]. Recent research focuses on the processing stages underlying speech production to determine the nature of speech motor control [6].
Recent studies have used the analysis of speech acoustic parameters to determine the nature of speech errors in patients with Broca’s aphasia [7, 8]. Acoustic speech analysis allows therapists to access information in speech signals, such as speech speed (rate), characteristics of vowel and consonant articulation, phonetic and prosodic aspects, and variability in the vocal tract shape [9]. Acoustic analysis of phonetic patterns of speech in patients with Broca’s aphasia in various studies shows deficits in motor control in these patients. These deficits include problems in timing, coordination in production movements, and laryngeal control, which is the result of deficits in coordination and integration of production movements and production execution necessary to produce the target segment [10].
During the production of vowels, each form of production creates a specific resonant frequency in the vocal cords, called a formant. Changes in the structure of the formant with different places of articulation are reflected by the frequencies of the formant at different times, which is called formant transition. Formant transition is based on changes in articulatory movement from consonant to vowel. Formant transition provides significant details about changes in the form of articulators. The transition time of the second formant (F2) reflects the changes in the articulatory movement over time, therefore this formant is used to reflect the timing of the articulatory movement [11].
In recent studies, formant transition in the speech of people with various neurological disorders has been analyzed [12, 13, 14]. The results of a study showed that the formant transition in people with cerebellar disease and bilateral damage to the upper motor neurons was longer than normal.
The results of various studies show that people with Broca’s aphasia display impairment in the timing and coordination of articulatory movement [15, 16]. To determine the nature of errors in people with Broca’s aphasia, various studies have examined the phonetic patterns of speech in these patients using acoustic analysis. The results of these studies show that people with Broca’s aphasia display impairment in the timing and coordination of articulatory movement. These studies examined the spectral properties of stop consonants through acoustic assessments. The results showed a change in the spectral characteristics of stop consonants in patients with Broca’s aphasia compared to the control group. This change in the spectral characteristics of stop consonants indicates that apart from the timing and coordination of the larynx with the vocal tract, patients with Broca’s aphasia have deficits in laryngeal control [15, 16].
Kurowski et al. [16] studied the articulation of nasal consonants in people with mild to moderate Broca’s aphasia and Wernicke’s aphasia. In this study, the formant transition of the nasal murmur was evaluated to examine speech timing. The range of the first harmony was also studied to evaluate articulation coordination, and the range of changes during the nasal murmur was examined to evaluate laryngeal control. The results showed patients with Broca’s aphasia had deficits in all three factors of laryngeal control, speech timing, and articulation coordination during the articulation of nasal consonants [16].
The most explicit and the most incontrovertible evidence for distinguishing between the stage of articulatory performance and the stages of phonological selection and planning comes from the acoustic research of speech articulation patterns. [5]. Deficits in any of the various aspects of motor control (timing, coordination of articulatory movements, laryngeal control) indicate that deficits in the structure of sounds in aphasic individuals are often due to impairment in articulatory performance rather than selection and planning of the target segment [5]. Considering that acoustic properties are different in diverse languages, language is considered a critical factor in the study of acoustics. Differences in acoustic properties of sounds in different languages have led to divergent results in the pattern of speech articulation in aphasic individuals reported by numerous studies [9, 17].
Most aphasiologists agree that aphasia is an impairment in language function and is caused due to brain damage; therefore it falls within the scope of neurologists [1]. However, in the context of learning language processing disorders under conditions, such as Broca traumatic aphasia, there is an opportunity to address relevant questions via a multidisciplinarity approach [1].
We’ve been studying the phonographic characteristics of resonant sounds in Persian spoken subjects with Broca’s aphasia, and an essential part of this investigation is to improve our knowledge of word emphasis given to patients suffering from Broca’s aphasia. Moreover, it can contribute to the development of applications for the evaluation and treatment of Broca’s aphasia among Persian-speaking patients as well as neurolinguistic research.
Determining the nature of errors in people with Broca’s aphasia, who constitute a vast portion of aphasia patients, is vital in identifying the underlying mechanisms involved in causing these errors. Diverse languages have different acoustic features and in Persian based on studies; it seems that no study has been conducted in this field. Considering the importance of the nature of errors and their mechanisms in aphasics, and also the role of acoustic assessments in determining the nature of errors accurately, this study aims to analyze formant transition in people with Broca’s aphasia to determine the nature of motor control speech and speech errors.
Materials and Methods
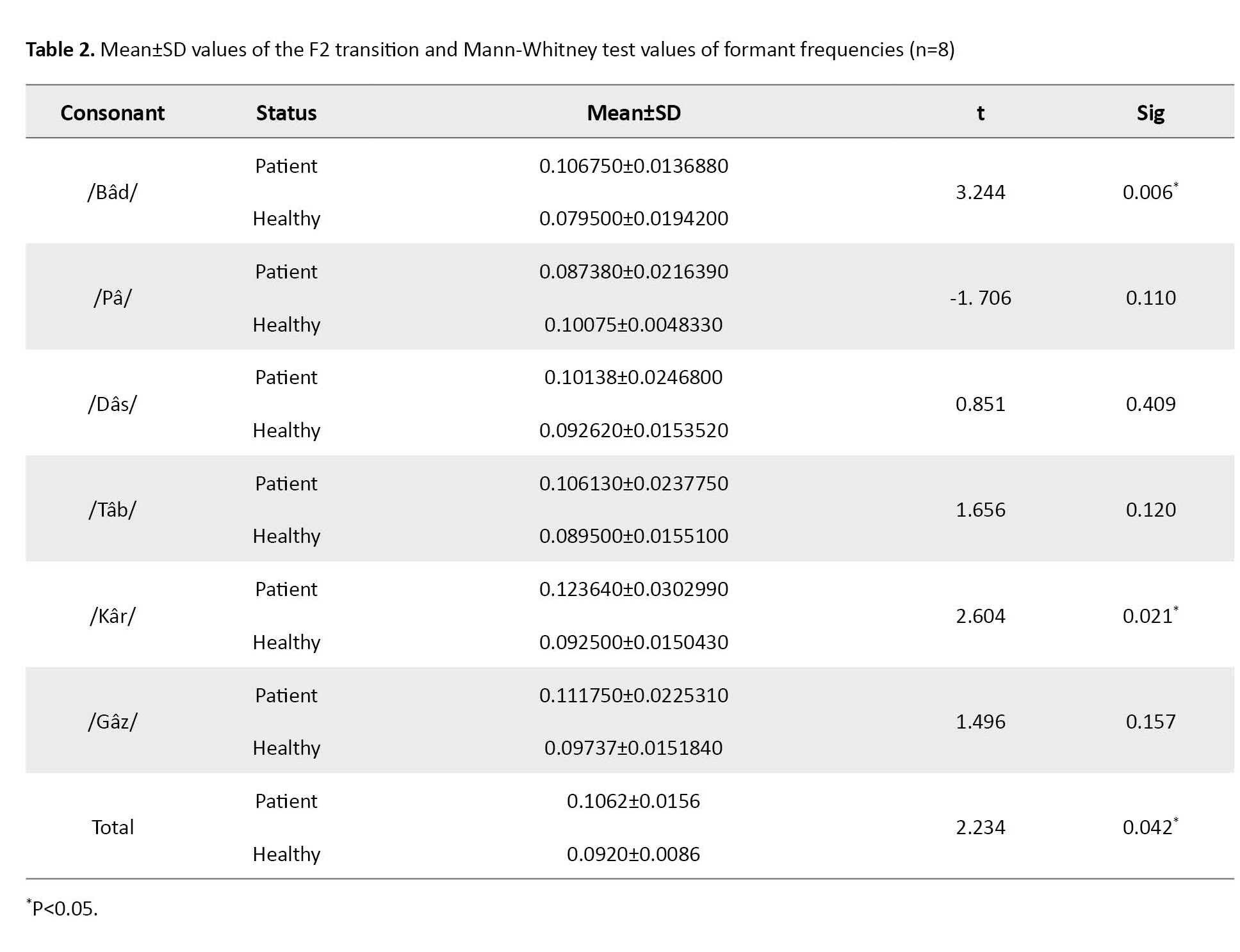
A total of 16 participants, a control group consisting of eight normal speakers, and eight patients with mild to moderate aphasia were selected for the experiments in this study. Most studies in this field have had about eight cases due to limitations in the number of Broca’s patients. All participants were selected voluntarily after completing the ethical consent form from Isfahan University of Medical Sciences. All participants were native Persian speakers. No history of psychiatric disorders or other cognitive impairments was observed. Hearing and vision were judged to be within normal limits. The aphasia group had a cerebrovascular accident (CVA) localized to the left hemisphere. They were at least 6 months post-cerebrovascular accident (CVA). Based on oral and verbal apraxia tests [18] and clinical examination, the patients had no co-occurring severe apraxia or dysarthria. The bedside version of the Persian WAB (P-WAB-1) [19] was used to determine the type and severity of aphasia. Finally, the subjects were eight males (50%) and eight females (50%). Based on the academic system in Iran, participants had one of three levels of education, elementary, diploma, or higher education. Table 1 presents demographic data for the participants.
Materials and procedure
Speech samples for the second formant transition included meaningful monosyllabic words with syllable structures (i.e. CV-CVC), beginning with the voiced and voiceless stop consonants (g-k-d-t-p-d). The words included / bâd / - / pâ / - / tâb / - / dâs / - / kâr / - / gâz /, which begin with bilabial, labiodental, and velar consonants in terms of place of articulation [20].
These words were written on 3×5 cm cards with legible and appropriate handwriting and presented to the participants. To minimize background noise (less than 50 dB), speech samples were recorded in a suitable noise-monitored environment (using the Praat software). To collect acoustic signals, after placing the participants in a suitable environment, the microphone was placed at a distance of 15 cm to the right of the participant’s mouth. In the next step, the participants were asked to read the target words written on the card clearly and naturally. If the participants were incapable of uttering each word correctly, the repetition task was used. To control the effect of loudness and speed of speech, the auditory model was pre-recorded in the repetition task and played a maximum of three times for each participant. If the participants were incapable of repetition and reading tasks, both tasks were used simultaneously. Voice recording was done simultaneously via a microphone (Micromic c520) and using a laptop (Sony VPCEA3S1E) equipped with a sound card. After recording, the spectrogram of each word was carefully examined using the Praat software, version 5.3.8.1.
Scoring and data analysis
The process of calculating F2 transition was investigated by the researcher and another expert outside the study so that the data collection and analysis process provided good reliability (60% of the samples). The intra-class correlation coefficient test was used to examine the correlation between the scores of the two testers and the Mann-Whitney test was used to compare aphasia and the normal group. Data were analyzed in the SPSS software, version 16 (SPSS Inc., Chicago, IL).
Results
In this study, due to some limitations in the sample size, the data distribution was more than the standard. To coordinate the distributions, the Mann-Whitney U test was used to compare the mean of the second formant transition in healthy and aphasic groups. It was the most appropriate choice due to the normality of the distribution of second formant transitions in each group and for each word.
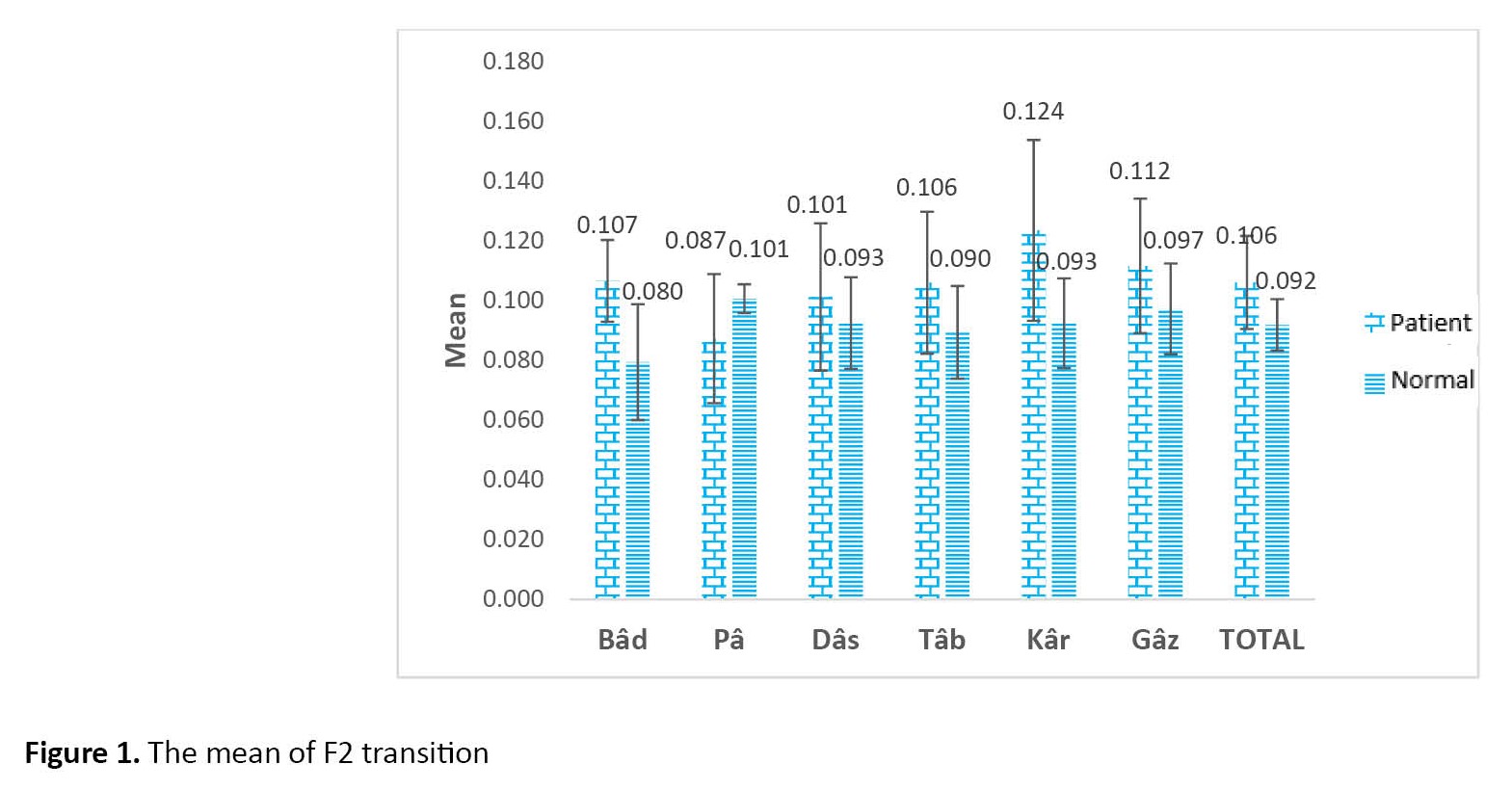
This study was conducted to evaluate the mean of the second formant transition of six words in eight people with aphasia and eight healthy individuals. The results show that the mean of the second formant transition in healthy people and people with aphasia are significantly different from each other in the words /Bâd/ and /Kâr/ (P<0.05), in which the mean of the second formant transition in both words was higher in the group of people with aphasia compared to healthy people. However, the mean of the second formant transition of the words /Pâ/, /Tâb/, /Gâz/, and /Dâs/ in aphasic individuals was not significantly different from healthy individuals.
The results also show that the mean of the second-formant transition in these six words in the group of aphasic people is significantly different compared to the group of healthy people (P<0.05), in which the mean of the second-formant transition in the group of people with aphasia is higher than healthy people (Table 2 and Figure 1).
Discussion
In the present study, the differences in the second formant transitions in people with mild to moderate aphasia and normal people were examined in one-syllable words: / bâd / - / pâ / - / tâb / - / dâs / - / kâr / - / gâz /. The results showed a significant difference between F2 transition in words / bâd / and /kâr/ in the two groups, while the mean score of second formant transition in words /pâ/, /tâb/, /gâz/ and /dâs/ in aphasic individuals was not significantly different from healthy individuals. The results also showed that the F2 transition in all six words was longer in the aphasic group than in the healthy group.
According to Fant’s study, the position and degree of constriction of the vocal tract play a critical role in vowel production and vary depending on the target vowels. The whole vocal cavity is involved in the production of vowels (e.g. lips, tongue, hard palate, pharyngeal wall, etc.). However, the place and degree of vocal tract constriction are mainly controlled by the position of the tongue [21, 22]. Due to the relationship between the position and degree of constriction of the vocal tract for vowels and formant frequencies, assumptions about a fundamental relationship between tongue position and formant frequency have been generalized in this area.
Acoustic research in neurological disorders has shown that the second formant transition is related to the clarity of speech of these clients. These patterns of damages to second formant transition in aphasic individuals are consistent with the results of studies conducted by Den Ouden et al. [12] and restrictions on the jaw, lips, and tongue movements and slow tongue movements appear to lead to prolonged second-formant transition and impaired timing of speech in aphasic individuals.
As mentioned earlier, no significant difference was observed in the mean of the second formant transition in the words /pâ/, /tâb/, /gâz/, and /dâs/ in the group of patients with aphasia compared to the healthy group. Significant differences in this study indicate that the second formant transition in voiced bilabial consonants and voiceless palatal consonants are prone to further damage compared to other consonants. These significant differences may be due to the greater demand for tongue movements and as a result, are more prone to damage in these people.
The second formant is chiefly determined by the position of the tongue (front to back) on the horizontal plane, especially for front vowels. Lips and pharyngeal sections of the vocal tract also play a part in F2 [23]. F2 variations show a compound effect of tongue height and advancement. Consequently, expanded F2 variations may reflect the inaccuracy of the horizontal position of the tongue in the production of vowels, while the increased absolute value of F2 indicates more frontal articulation patterns in people with aphasia.
The results of a study conducted by Lee et al. [23] indicated that different tissues show different sensitivities to speech-motor control problems.
Considering that F2 transition depends on the type of tongue movement from the consonant position to the vowel and also tongue muscles are one of the smallest and most delicate muscles in the body, it can be concluded that the brain can control speech movement.
The acoustic distinction between emphatic aphasia and plain aphasia cannot be achieved by subjects with Broca’s aphasia. This is because such patients are unable to coordinate the articulatory sets needed to create sound patterns that induce loud sounds.
As a consequence, subjects with Broca aphasia have demonstrated articulatory limitations in their tongue movements to the right and therefore produce an emphatic sound at the same place of articulation as plain speech.
Compared to the normal speakers, subjects with Broca aphasia have shown higher F2 values. Focusing on Broca’s aphasia provides an insight into its characteristics and indicates difficulties in applying the secondary articulation to a sufficient extent. Furthermore, the results of these studies can be used to develop assessment techniques and therapeutic programs for Persian speakers with Broca’s aphasia [24, 25].
Ethical Considerations
Compliance with ethical guidelines
The Ethics Committee of Isfahan University of Medical Sciences approved the study (Code: 14828).
Funding
This paper was extracted from the MSc thesis of Atefe Qodrati Tavana, Department of English Language, Islamic Azad University, Isfahan (Khorasgan) Branch.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
We gratefully acknowledge the individuals who voluntarily participated in this study.
References
Aphasia is an acquired language communication disorder caused by stroke, tumor, or brain injury characterized by a disorder in language skills, such as speaking, comprehension, reading, and writing. Different types of aphasia with diverse clinical features exist. Broca’s aphasia is one of the most familiar and widespread types of aphasia, classified as non-fluent aphasia. The prominent features of speech in people with Broca’s aphasia include limited and non-fluent verbal output produced with many efforts and pauses, reduced speech rate, reduced speech length, inaccurate production of sounds, agrammatism, and impaired speech tone [1]. One of the clinical manifestations of Broca’s aphasia is a deficit in speech articulation. Various studies have shown that patients with Broca’s aphasia suffer from deficits that affect the structure of sounds in their speech output. Determining the nature of speech errors in these patients is strictly necessary and crucial in diagnosing the disturbed and effective mechanisms that cause these errors [2].
On the other hand, the production of speech sounds requires the integration of auditory, somatosensory, and motor information represented in the temporal, parietal, and frontal lobes. In addition to subcortical structures, cortical areas, and their functional connections form a functional unit called the speech-motor control system [3]. Speech motor control refers to systems and strategies which control speech production. Some sets of productive movements convert the desired language message into an acoustic signal interpreted by the listener, including the input of the speech motor control system, linguistic representations, especially the sequence of abstract units, i.e. phonemes, and also the output of the speech motor control system. Therefore, the speech motor control system is located between the two processes of language formulation and the creation of an acoustic signal that transmits the message [4, 5]. Recent research focuses on the processing stages underlying speech production to determine the nature of speech motor control [6].
Recent studies have used the analysis of speech acoustic parameters to determine the nature of speech errors in patients with Broca’s aphasia [7, 8]. Acoustic speech analysis allows therapists to access information in speech signals, such as speech speed (rate), characteristics of vowel and consonant articulation, phonetic and prosodic aspects, and variability in the vocal tract shape [9]. Acoustic analysis of phonetic patterns of speech in patients with Broca’s aphasia in various studies shows deficits in motor control in these patients. These deficits include problems in timing, coordination in production movements, and laryngeal control, which is the result of deficits in coordination and integration of production movements and production execution necessary to produce the target segment [10].
During the production of vowels, each form of production creates a specific resonant frequency in the vocal cords, called a formant. Changes in the structure of the formant with different places of articulation are reflected by the frequencies of the formant at different times, which is called formant transition. Formant transition is based on changes in articulatory movement from consonant to vowel. Formant transition provides significant details about changes in the form of articulators. The transition time of the second formant (F2) reflects the changes in the articulatory movement over time, therefore this formant is used to reflect the timing of the articulatory movement [11].
In recent studies, formant transition in the speech of people with various neurological disorders has been analyzed [12, 13, 14]. The results of a study showed that the formant transition in people with cerebellar disease and bilateral damage to the upper motor neurons was longer than normal.
The results of various studies show that people with Broca’s aphasia display impairment in the timing and coordination of articulatory movement [15, 16]. To determine the nature of errors in people with Broca’s aphasia, various studies have examined the phonetic patterns of speech in these patients using acoustic analysis. The results of these studies show that people with Broca’s aphasia display impairment in the timing and coordination of articulatory movement. These studies examined the spectral properties of stop consonants through acoustic assessments. The results showed a change in the spectral characteristics of stop consonants in patients with Broca’s aphasia compared to the control group. This change in the spectral characteristics of stop consonants indicates that apart from the timing and coordination of the larynx with the vocal tract, patients with Broca’s aphasia have deficits in laryngeal control [15, 16].
Kurowski et al. [16] studied the articulation of nasal consonants in people with mild to moderate Broca’s aphasia and Wernicke’s aphasia. In this study, the formant transition of the nasal murmur was evaluated to examine speech timing. The range of the first harmony was also studied to evaluate articulation coordination, and the range of changes during the nasal murmur was examined to evaluate laryngeal control. The results showed patients with Broca’s aphasia had deficits in all three factors of laryngeal control, speech timing, and articulation coordination during the articulation of nasal consonants [16].
The most explicit and the most incontrovertible evidence for distinguishing between the stage of articulatory performance and the stages of phonological selection and planning comes from the acoustic research of speech articulation patterns. [5]. Deficits in any of the various aspects of motor control (timing, coordination of articulatory movements, laryngeal control) indicate that deficits in the structure of sounds in aphasic individuals are often due to impairment in articulatory performance rather than selection and planning of the target segment [5]. Considering that acoustic properties are different in diverse languages, language is considered a critical factor in the study of acoustics. Differences in acoustic properties of sounds in different languages have led to divergent results in the pattern of speech articulation in aphasic individuals reported by numerous studies [9, 17].
Most aphasiologists agree that aphasia is an impairment in language function and is caused due to brain damage; therefore it falls within the scope of neurologists [1]. However, in the context of learning language processing disorders under conditions, such as Broca traumatic aphasia, there is an opportunity to address relevant questions via a multidisciplinarity approach [1].
We’ve been studying the phonographic characteristics of resonant sounds in Persian spoken subjects with Broca’s aphasia, and an essential part of this investigation is to improve our knowledge of word emphasis given to patients suffering from Broca’s aphasia. Moreover, it can contribute to the development of applications for the evaluation and treatment of Broca’s aphasia among Persian-speaking patients as well as neurolinguistic research.
Determining the nature of errors in people with Broca’s aphasia, who constitute a vast portion of aphasia patients, is vital in identifying the underlying mechanisms involved in causing these errors. Diverse languages have different acoustic features and in Persian based on studies; it seems that no study has been conducted in this field. Considering the importance of the nature of errors and their mechanisms in aphasics, and also the role of acoustic assessments in determining the nature of errors accurately, this study aims to analyze formant transition in people with Broca’s aphasia to determine the nature of motor control speech and speech errors.
Materials and Methods
A total of 16 participants, a control group consisting of eight normal speakers, and eight patients with mild to moderate aphasia were selected for the experiments in this study. Most studies in this field have had about eight cases due to limitations in the number of Broca’s patients. All participants were selected voluntarily after completing the ethical consent form from Isfahan University of Medical Sciences. All participants were native Persian speakers. No history of psychiatric disorders or other cognitive impairments was observed. Hearing and vision were judged to be within normal limits. The aphasia group had a cerebrovascular accident (CVA) localized to the left hemisphere. They were at least 6 months post-cerebrovascular accident (CVA). Based on oral and verbal apraxia tests [18] and clinical examination, the patients had no co-occurring severe apraxia or dysarthria. The bedside version of the Persian WAB (P-WAB-1) [19] was used to determine the type and severity of aphasia. Finally, the subjects were eight males (50%) and eight females (50%). Based on the academic system in Iran, participants had one of three levels of education, elementary, diploma, or higher education. Table 1 presents demographic data for the participants.

Materials and procedure
Speech samples for the second formant transition included meaningful monosyllabic words with syllable structures (i.e. CV-CVC), beginning with the voiced and voiceless stop consonants (g-k-d-t-p-d). The words included / bâd / - / pâ / - / tâb / - / dâs / - / kâr / - / gâz /, which begin with bilabial, labiodental, and velar consonants in terms of place of articulation [20].
These words were written on 3×5 cm cards with legible and appropriate handwriting and presented to the participants. To minimize background noise (less than 50 dB), speech samples were recorded in a suitable noise-monitored environment (using the Praat software). To collect acoustic signals, after placing the participants in a suitable environment, the microphone was placed at a distance of 15 cm to the right of the participant’s mouth. In the next step, the participants were asked to read the target words written on the card clearly and naturally. If the participants were incapable of uttering each word correctly, the repetition task was used. To control the effect of loudness and speed of speech, the auditory model was pre-recorded in the repetition task and played a maximum of three times for each participant. If the participants were incapable of repetition and reading tasks, both tasks were used simultaneously. Voice recording was done simultaneously via a microphone (Micromic c520) and using a laptop (Sony VPCEA3S1E) equipped with a sound card. After recording, the spectrogram of each word was carefully examined using the Praat software, version 5.3.8.1.
Scoring and data analysis
The process of calculating F2 transition was investigated by the researcher and another expert outside the study so that the data collection and analysis process provided good reliability (60% of the samples). The intra-class correlation coefficient test was used to examine the correlation between the scores of the two testers and the Mann-Whitney test was used to compare aphasia and the normal group. Data were analyzed in the SPSS software, version 16 (SPSS Inc., Chicago, IL).
Results
In this study, due to some limitations in the sample size, the data distribution was more than the standard. To coordinate the distributions, the Mann-Whitney U test was used to compare the mean of the second formant transition in healthy and aphasic groups. It was the most appropriate choice due to the normality of the distribution of second formant transitions in each group and for each word.
This study was conducted to evaluate the mean of the second formant transition of six words in eight people with aphasia and eight healthy individuals. The results show that the mean of the second formant transition in healthy people and people with aphasia are significantly different from each other in the words /Bâd/ and /Kâr/ (P<0.05), in which the mean of the second formant transition in both words was higher in the group of people with aphasia compared to healthy people. However, the mean of the second formant transition of the words /Pâ/, /Tâb/, /Gâz/, and /Dâs/ in aphasic individuals was not significantly different from healthy individuals.
The results also show that the mean of the second-formant transition in these six words in the group of aphasic people is significantly different compared to the group of healthy people (P<0.05), in which the mean of the second-formant transition in the group of people with aphasia is higher than healthy people (Table 2 and Figure 1).

Discussion
In the present study, the differences in the second formant transitions in people with mild to moderate aphasia and normal people were examined in one-syllable words: / bâd / - / pâ / - / tâb / - / dâs / - / kâr / - / gâz /. The results showed a significant difference between F2 transition in words / bâd / and /kâr/ in the two groups, while the mean score of second formant transition in words /pâ/, /tâb/, /gâz/ and /dâs/ in aphasic individuals was not significantly different from healthy individuals. The results also showed that the F2 transition in all six words was longer in the aphasic group than in the healthy group.
According to Fant’s study, the position and degree of constriction of the vocal tract play a critical role in vowel production and vary depending on the target vowels. The whole vocal cavity is involved in the production of vowels (e.g. lips, tongue, hard palate, pharyngeal wall, etc.). However, the place and degree of vocal tract constriction are mainly controlled by the position of the tongue [21, 22]. Due to the relationship between the position and degree of constriction of the vocal tract for vowels and formant frequencies, assumptions about a fundamental relationship between tongue position and formant frequency have been generalized in this area.
Acoustic research in neurological disorders has shown that the second formant transition is related to the clarity of speech of these clients. These patterns of damages to second formant transition in aphasic individuals are consistent with the results of studies conducted by Den Ouden et al. [12] and restrictions on the jaw, lips, and tongue movements and slow tongue movements appear to lead to prolonged second-formant transition and impaired timing of speech in aphasic individuals.
As mentioned earlier, no significant difference was observed in the mean of the second formant transition in the words /pâ/, /tâb/, /gâz/, and /dâs/ in the group of patients with aphasia compared to the healthy group. Significant differences in this study indicate that the second formant transition in voiced bilabial consonants and voiceless palatal consonants are prone to further damage compared to other consonants. These significant differences may be due to the greater demand for tongue movements and as a result, are more prone to damage in these people.
The second formant is chiefly determined by the position of the tongue (front to back) on the horizontal plane, especially for front vowels. Lips and pharyngeal sections of the vocal tract also play a part in F2 [23]. F2 variations show a compound effect of tongue height and advancement. Consequently, expanded F2 variations may reflect the inaccuracy of the horizontal position of the tongue in the production of vowels, while the increased absolute value of F2 indicates more frontal articulation patterns in people with aphasia.
The results of a study conducted by Lee et al. [23] indicated that different tissues show different sensitivities to speech-motor control problems.
Considering that F2 transition depends on the type of tongue movement from the consonant position to the vowel and also tongue muscles are one of the smallest and most delicate muscles in the body, it can be concluded that the brain can control speech movement.
The acoustic distinction between emphatic aphasia and plain aphasia cannot be achieved by subjects with Broca’s aphasia. This is because such patients are unable to coordinate the articulatory sets needed to create sound patterns that induce loud sounds.
As a consequence, subjects with Broca aphasia have demonstrated articulatory limitations in their tongue movements to the right and therefore produce an emphatic sound at the same place of articulation as plain speech.
Compared to the normal speakers, subjects with Broca aphasia have shown higher F2 values. Focusing on Broca’s aphasia provides an insight into its characteristics and indicates difficulties in applying the secondary articulation to a sufficient extent. Furthermore, the results of these studies can be used to develop assessment techniques and therapeutic programs for Persian speakers with Broca’s aphasia [24, 25].
Ethical Considerations
Compliance with ethical guidelines
The Ethics Committee of Isfahan University of Medical Sciences approved the study (Code: 14828).
Funding
This paper was extracted from the MSc thesis of Atefe Qodrati Tavana, Department of English Language, Islamic Azad University, Isfahan (Khorasgan) Branch.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
We gratefully acknowledge the individuals who voluntarily participated in this study.
References
- LaPointe L. Aphasia and related neurogenic language disorders. Stuttgart: Thieme Publishers Series; 2011. [Link]
- Kirshner HS, Wilson SM. Aphasia and aphasic syndromes. In: Jankovic J, editor. Bradley’s Neurology in Clinical Practice E-Book. Amsterdam: Elsevier; 2021. [Link]
- Guenther FH, Vladusich T. A neural theory of speech acquisition and production. J Neurolinguistics. 2012; 25(5):408-22. [DOI:10.1016/j.jneuroling.2009.08.006] [PMID] [PMCID]
- Staiger A, Schölderle T. Speech motor control: Current perspectives on development and disorders. Basel: Karger; 2019. [DOI:10.1159/978-3-318-06532-9]
- Ziegler W. Phonology versus phonetics in speech sound disorders. In: Maassen B, Kent R, Peters H, editors. Speech motor control in normal and disordered speech. Rockville: ASHA Press; 2016. [Link]
- Mailend ML, Maas E, Beeson PM, Story BH, Forster KI. Speech motor planning in the context of phonetically similar words: Evidence from apraxia of speech and aphasia. Neuropsychologia. 2019; 127:171-84. [DOI:10.1016/j.neuropsychologia.2019.02.018] [PMID] [PMCID]
- Gillespie S, Laures-Gore J, Moore E, Farina M, Russell S, Haaland B. Identification of affective state change in adults with aphasia using speech acoustics. J Speech Lang Hear Res. 2018; 61(12):2906-16. [DOI:10.1044/2018_JSLHR-S-17-0057] [PMID] [PMCID]
- Niziolek CA, Kiran S. Assessing speech correction abilities with acoustic analyses: Evidence of preserved online correction in persons with aphasia. Int J Speech Lang Pathol. 2018; 20(6):659-68. [DOI:10.1080/17549507.2018.1498920] [PMID] [PMCID]
- McNeil IM. Clinical management of sensorimotor speech disorders. New York, NY: Thieme; 2018. [Link]
- Verhaegen C, Delvaux V, Fagniart S, Huet K, Piccaluga M, Harmegnies B. Phonological and phonetic impairment in aphasic speech: an acoustic study of the voice onset time of six French-speaking aphasic patients. Clin Linguist Phon. 2020; 34(3):201-21. [DOI:10.1080/02699206.2019.1619095] [PMID]
- Ball MJ, Müller N. Phonetics for communication disorders. New York: Psychology Press; 2013. [DOI:10.4324/9781315805573]
- den Ouden DB, Galkina E, Basilakos A, Fridriksson J. Vowel formant dispersion reflects severity of apraxia of speech. Aphasiology. 2018; 32(8):902-21. [DOI:10.1080/02687038.2017.1385050] [PMID] [PMCID]
- Maas E, Mailend ML, Guenther FH. Feedforward and feedback control in apraxia of speech: Effects of noise masking on vowel production. J Speech Lang Hear Res. 2015; 58(2):185-200. [DOI:10.1044/2014_JSLHR-S-13-0300] [PMID] [PMCID]
- Jacks A. Bite block vowel production in apraxia of speech. J Speech Lang Hear Res. 2008; 51(4):898-913. [DOI:10.1044/1092-4388(2008/066)] [PMID]
- Sarno MT. Recovery and rehabilitation in aphasia. In: Sarno MT, editor. Acquired aphasia. Massachusetts: Academic Press; 1998. [DOI:10.1016/B978-012619322-0/50021-X]
- Kurowski KM, Blumstein SE, Palumbo CL, Waldstein RS, Burton MW. Nasal consonant production in Broca’s and Wernicke’s aphasics: Speech deficits and neuroanatomical correlates. Brain Lang. 2007; 100(3):262-75. [DOI:10.1016/j.bandl.2006.10.002] [PMID] [PMCID]
- Hatfield FM, Walton K. Phonological patterns in a case of aphasia. Lang Speech. 1975; 18(4):341-57. [DOI:10.1177/002383097501800405] [PMID]
- Yadegari F. Oral and verbal apraxia tasks for adults. Tehran: University of Social Welfare and Rehabilitation Sciences; 2011.
- Nilipour R, Pourshahbaz A, Ghoreyshi ZS. Reliability and validity of bedside version of Persian WAB (P-WAB-1). Basic Clin Neurosci. 2014; 5(4):253-8. [PMID]
- Abnavi F, Ghasisin L, Alinejad B, Mahaki B. [Acoustic analysis of speech timing of individuals with Broca’s aphasia in nasal consonant production (Persian)]. Middle East J Disabil Stud. 2014; 4(2):33-42. [Link]
- Stevens KN, House AS. Development of a quantitative description of vowel articulation. J Acoust Soc Am. 1955; 27(3):484-93. [DOI:10.1121/1.1907943]
- Fant G. Acoustic theory of speech production. Netherlands The Hague University; 1960.
- Lee J, Shaiman S, Weismer G. Relationship between tongue positions and formant frequencies in female speakers. J Acoust Soc Am. 2016; 139(1):426-40. [DOI:10.1121/1.4939894] [PMID]
- Weismer G, Jeng JY, Laures JS, Kent RD, Kent JF. Acoustic and intelligibility characteristics of sentence production in neurogenic speech disorders. Folia Phoniatr Logop. 2001; 53(1):1-18. [PMID]
- Tjaden K, Wilding GE. Rate and loudness manipulations in dysarthria: Acoustic and perceptual findings. J Speech Lang Hear Res. 2004; 47(4):766-83. [DOI:10.1044/1092-4388(2004/058)] [PMID]
Type of Study: Research |
Subject:
Speech Therapy
Received: 2023/01/19 | Accepted: 2023/06/12 | Published: 2022/02/6
Received: 2023/01/19 | Accepted: 2023/06/12 | Published: 2022/02/6