Volume 5, Issue 1 (Continuously Updated 2022)
Func Disabil J 2022, 5(1): 0-0 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Asharlous A, Riazi A, Janani S, Rajabi S, Rakhshan A, Khabazkhoob M, et al . Refractive Error and Ocular Biometric Changes in the Treatment of Diabetes Mellitus. Func Disabil J 2022; 5 (1) : 60
URL: http://fdj.iums.ac.ir/article-1-192-en.html
URL: http://fdj.iums.ac.ir/article-1-192-en.html
Amir Asharlous1 

 , Abbas Riazi1
, Abbas Riazi1 

 , Samira Janani1
, Samira Janani1 

 , Sattar Rajabi *2
, Sattar Rajabi *2 

 , Amir Rakhshan3
, Amir Rakhshan3 

 , Mehdi Khabazkhoob4
, Mehdi Khabazkhoob4 

 , Zahra Sadat Dibaji Forooshani5
, Zahra Sadat Dibaji Forooshani5 

 , Hamed Tabesh6
, Hamed Tabesh6 




 , Abbas Riazi1
, Abbas Riazi1 

 , Samira Janani1
, Samira Janani1 

 , Sattar Rajabi *2
, Sattar Rajabi *2 

 , Amir Rakhshan3
, Amir Rakhshan3 

 , Mehdi Khabazkhoob4
, Mehdi Khabazkhoob4 

 , Zahra Sadat Dibaji Forooshani5
, Zahra Sadat Dibaji Forooshani5 

 , Hamed Tabesh6
, Hamed Tabesh6 


1- Department of Optometry, Rehabilitation Research Center, School of Rehabilitation Sciences, Iran University of Medical Sciences, Tehran, Iran.
2- Department of Optometry, Rehabilitation Research Center, School of Rehabilitation Sciences, Iran University of Medical Sciences, Tehran, Iran. ,sattar.rajabi@yahoo.com
3- Department of Foreign Languages, Faculty of Foreign Languages and Literatures, Tehran University of Medical Sciences, Tehran, Iran.
4- Department of Basic Sciences, School of Nursing and Midwifery, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
5- Bank Melli Hospital, Tehran, Iran.
6- Department of Medical Informatics, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
2- Department of Optometry, Rehabilitation Research Center, School of Rehabilitation Sciences, Iran University of Medical Sciences, Tehran, Iran. ,
3- Department of Foreign Languages, Faculty of Foreign Languages and Literatures, Tehran University of Medical Sciences, Tehran, Iran.
4- Department of Basic Sciences, School of Nursing and Midwifery, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
5- Bank Melli Hospital, Tehran, Iran.
6- Department of Medical Informatics, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
Full-Text [PDF 1066 kb]
(613 Downloads)
| Abstract (HTML) (1584 Views)
Full-Text: (802 Views)
Introduction
According to the International Diabetes Federation in 2017, 451 million people worldwide had diabetes, and this number is projected to reach 693 million by 2045 [1]. Diabetes is the most common endocrine disease in countries; [2] and hyperglycemia is an essential feature, and a risk factor for its formation; [3] hemoglobin glycate is the most valid laboratory test to assess blood sugar [4]. Acute and chronic changes in blood sugar cause changes in the structure of the eye, such as fluctuations in near and far vision and ocular biometric parameters [5, 6]. It is essential to be aware of the current situation and predict possible changes in the individual’s treatment path in making decisions about the possibility of making changes in the individual’s optical correction.
Various studies have been performed to evaluate the refractive error in people with diabetes compared to normal people [5, 7, 8] and its changes after being in the process of hypoglycemia [9-11] so that the common finding of them is Myopia shift in people with uncontrolled blood sugar [12] and hyperopic shift after being in the process of hypoglycemia [10]. However, sometimes the opposite results have been presented in studies; among them, it is possible to mention the slight shift of hyperopia after transient hyperglycemia, [13] no change in refraction during blood sugar fluctuation, [14, 15] and myopic shift during hyperglycemia reduction [16].
A set of biometric factors, such as axial length, corneal power, and crystalline thickness of the lens, along with the refractive index of the cornea, lens, aqueous humor, and vitreous, determine the refractive error of the eye, [17] therefore any change in these parameters due to reduced blood sugar may cause changes in the refractive error changes in the eyes of people with diabetes. Among the most critical changes in biometric parameters while changes in blood sugar are changes in the structure and dimensions of the crystalline lens, [18] whose related changes in blood sugar are controversial [19]. Axial length is also considered to be an essential component associated with refractive error changes and diabetic retinopathy [20].
In the present study, while examining a group of people with hemoglobin glycate of more than 7.5%, the changes in biometric parameters, the components of refractive error, and the relationship between them with blood sugar reduction are evaluated.
Materials and Methods
Participants
The present study was conducted at the Iran University of Medical Sciences in 2020. People with the first diagnosis of type 2 diabetes or a history of poor blood sugar control were evaluated. In addition, the inclusion criteria included having hemoglobin glycate of more than 7.5% and the absence of any ocular and systemic diseases, including abnormal retinal and corneal manifestations, eye pressure of more than 21 mm Hg, hypertension, hyperlipidemia, anemia, history of any eye surgery, and long-term use of systemic and topical steroids.
Examinations
People with hemoglobin glycate of more than seven and a half were referred to the optometry department for evaluation and eye examinations by an internal medicine specialist. Eye examinations of the anterior and posterior segments were performed using a slit lamp (Haag-Streit corp., Switzerland) with a 90-diopter Volk lens to evaluate the presence criteria.
For patients who met the inclusion criteria, refraction and biometrics were recorded from both eyes. First, uncorrected visual acuity was recorded by the Snllen chart with logMAR criteria. Then, objective refraction was performed by Tono ref 2 (Autoref/Kerato/Tonometer) Nidek, Japan, and the results were confirmed by a retinoscope (Heine Beta 200, Heine Optotechnik, Germany). It should be noted that spherical components, spherical equivalents, and J0 and J45 parameters were recorded based on objective values. In the next step, ocular biometric evaluation was performed by IOLMaster 700 (Carl Zeiss Meditec, Jena, Germany), and the parameters of axial length, anterior chamber depth, and crystalline lens thickness were measured, and biometric and refraction data sets were analyzed.
The process of treatment and reduction of blood sugar was started under the supervision of an internal medicine specialist, and all the above measurements were recorded along with hemoglobin glycated level after one and a half and three months from the beginning of the process of treatment of hypoglycemia; finally, statistical analysis was performed on the findings after recording all the information. It should be noted that all measurements in each examination were performed on the same day as the measurement of hemoglobin glycate.
Statistical analysis
The present study presents descriptive information, including Mean±SD, and 95% confidence interval for all parameters.
Vector analysis to examine the change in astigmatism considering (S [sphere], C [cylinder], α [axis]) and calculating J0=-(C/2)×cos(2α), J45=-(C/2) sin(2α) and SE=S+C/2 were performed [20].
Analytical analysis was performed after examining the normality of data distribution by the Kolmogorov-Smirnov test. The mean differences of each parameter in the three examinations were checked by repeated-measures analysis of variance (ANOVA), and if the difference was significant, pair-wise differences were evaluated by Bonferroni correction. Due to the correlation of data between the two eyes, to analyze the relationship between changes in each parameter while changes in blood sugar between the three examinations during blood sugar control were examined by generalized estimating equation (GEE) analysis. It should be noted that all statistical analyzes were performed by SPSS software, version 25 (IBM corporation, Armonk, N.Y.) at a significant level of 5%.
Results
In the present study, 60 eyes of 30 patients with a Mean±SD age of 51.63±6.79 years (25 to 60 years) were evaluated so that the number of people in both gender groups was equal.
Based on the information in Table 1, which shows the amount of hemoglobin glycate measured in each examination, it can be seen that this parameter has decreased by an average of 1.028% compared to the baseline measurement in the third month (P<0.001).
.png)
The process of lowering blood sugar was such that hemoglobin glycated levels were different from each other in the first and second examinations (P<0.001), first and third (P<0.001), and second and third (P=0.003). It should be mentioned in tables FU1means Follow-up 1.5 months and FU2 means Follow-up 3 months.
ANOVA with repeated measures showed no difference between spherical and spherical mean in three examinations (all, P>0.05) so that the spherical equivalent in the three examinations was -0.20±-1.55, -0.25±-1.55, and -0.25±-1.54 diopters, respectively (P=0.340). Also, despite the difference in the numerical value of the parameters J0 (P=0.143) and J45 (P=0.910) in each examination, this discrepancy was not statistically significant (P>0.05), Table 2 presents these data.
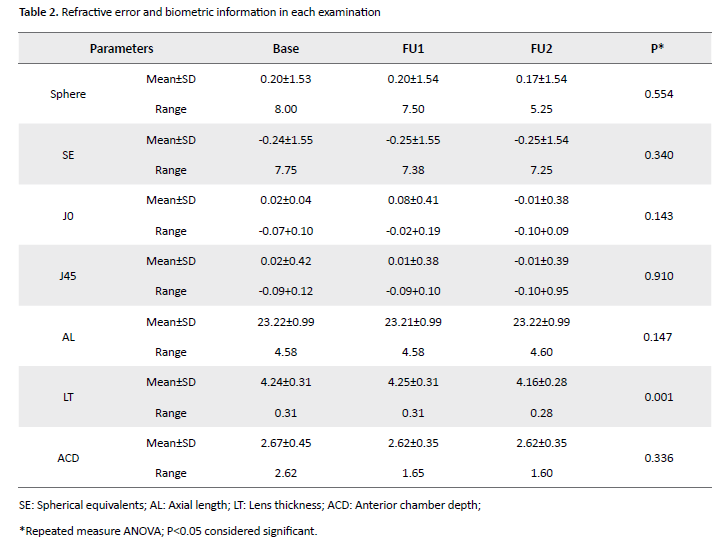
Among the studied biometric parameters (Table 2), only the average thickness of the crystalline lens was different between the three examinations (P=0.001) so that pair-wise evaluation showed the difference in the crystalline lens thickness between the first and third examinations (P=0.009, MD=+0.52 mm) and between the second and third examination (P=0.005, MD=+0.64 mm). The evaluation of mean values of axial length parameters (P=0.147) and anterior chamber depth (P=0.336) did not differ significantly between the three evaluations.
Finally, GEE analysis showed that among all the studied parameters (Table 3), the axial length of the eyeball changed while lowering blood sugar.
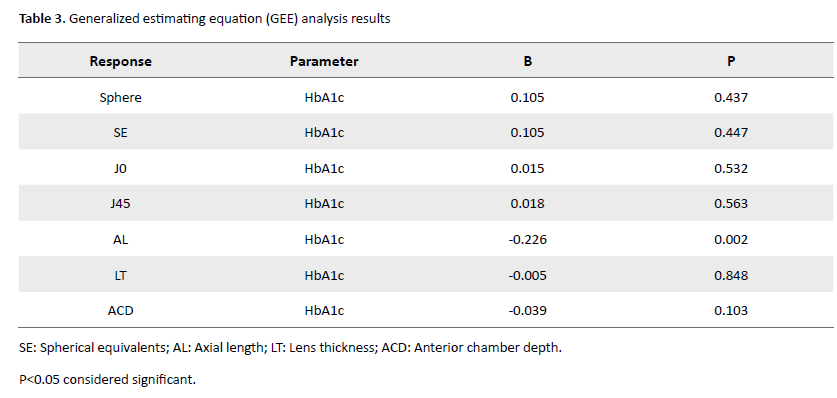
In fact, despite the lack of difference in the mean axial length in the three examinations (P=0.147), a 1% decrease in hemoglobin glycate increased the axial length by 0.226 mm (P=0.002); GEE analysis showed no significant relationship between changes in other parameters, including refractive error components, lens thickness, and anterior chamber depth with glycemic control (all, P>0.05).
Discussion
Being on the path of lowering blood sugar is the crucial action after the diagnosis of diabetes, which is one of the unwanted side effects in some people; [10] therefore it is recommended to avoid any optical prescription in conditions where the blood sugar level is abnormally high or low [12]. Now the answer to how long to wait after treatment is to record the stability of refractive changes is clinically significant.
The present study results show no change in the spherical, cylindrical, and spherical equivalent and a 1% decrease in hemoglobin glycate within three months after the start of diabetic treatment. The exact mechanism of refractive error changes in blood glucose changes has not yet been elucidated well. However, some previous studies have shown that severe and sudden glycemic decrease leads to hyperopic shifts in refraction [9, 11]. Peak shift in hyperopia has been observed in different studies on the seventh day (Li et al.), [21] tenth day (Okamoto et al.), [10] between 7 to 14 days (Saito et al.), [22] and finally on the seventeenth day (Lin et al.) [9] after starting treatment.
The hyperopic shift created in the process of glycemic control gradually decreases, and returning to baseline level has also been mentioned in different studies, such as in the study of Li et al. This time was in two to four weeks after starting treatment [21] and Okamoto et al.’ study reported 14 to 84 days after the initial evaluation. [10].
The set of results of the mentioned studies along with the results of the present study can be interpreted as one of the reasons for the lack of difference in refractive error values observed between the baseline and the first examination, due to the time difference of one and a half months between the two examinations. Therefore, the created hyperopic shift will be expected to return to the base level from this period. It has also been shown a direct relationship between the rate of maximal hyperopic shift and the timing of peak changes since the start of glycemic control treatment [10].
On the other hand, the blood sugar level of the evaluated individuals also affected the observed results so that Ebeigbe found that the transient refractive error changes in newly diagnosed diabetic patients depended on blood glucose levels [23]. The mean of hemoglobin glycate in the pre-treatment evaluation and the maximum changes in refraction in the study of Li et al. was 12.20±1.50% and 1.6 diopters of hyperopic shift, respectively [21]. In Okamoto et al., it was equal to 11.90±2.8% and 1.47 diopters of hyperopic shift [10] and in the present study, it was equal to 8.82±1.31% without refractive error changes. According to the above, it can be concluded that the lower blood sugar level of the subjects in the present study compared to other studies is also one of the compelling reasons for not observing changes in refractive error in the examination before and after the person’s presence in the process of reduction of blood sugar.
Therefore, it is predictable that even if you do not know the status of the refractive error line, you can know the amount of hemoglobin glycate in the person before starting treatment for hypoglycemia, the number of days elapsed since the start of treatment, and the amount of decreased blood glucose. Thus, we gained valuable information about the individual’s condition and decided on an optical prescription based on pre-treatment data.
Evaluation of biometric parameters in the present study showed that the average crystal lens thickness in the third examination is lower than the first and the third examination than the second. However, the results of GEE analysis show that the differences observed in this parameter cannot be directly attributed to the changes in blood sugar. Decreasing the crystalline lens thickness in the process of lowering blood sugar is a common finding that has been mentioned in various studies [10, 19]. Besides, Huntjens et al. observed that a 2% short-term increase in glycated hemoglobin caused a 0.21 mm increase in the crystalline thickness of the lens [24]. However, in some previous studies, differences in this parameter in the treatment process of diabetes [11] and also in the comparison of people with type 2 diabetes and normal people [15] were not significant; it may be inferred that the rate of change in hemoglobin glycated was the cause of the observed difference in crystalline lens thickness changes in the process of glycemic control.
Also, the results of the present study showed no change in the average anterior chamber depth while reducing blood sugar. Lack of difference in the depth of the anterior chamber of the eyeball while lowering blood sugar is a common finding reported in various studies. [10, 25] However, in their study, Kocaturk et al. found that the average anterior chamber depth in the right eye of people with uncontrolled type 2 diabetes was lower than in normal people; this difference was not observed in the left eye of the same individuals [26].
It is commonly accepted that reducing the crystalline thickness of the lens increases the depth of the anterior chamber of the eyeball [27]. Therefore, it is expected that the average thickness of the crystalline lens in the third month after the start of treatment compared to the baseline was associated with a greater depth of the anterior chamber. Two possible reasons exist for this, the first reason is that changes in corneal curvature may have occurred during treatment, neutralizing the effect of changes in lens thickness on anterior chamber depth, or that the convexity of the lens may have shifted further toward the vitreous, which has not affected the depth of the anterior chamber [28].
The results of the present study show no difference in the mean axial length of the eyeball measured between the three examinations. This finding was similarly observed in the studies of Li et al. and Seven et al. [11, 21] However, GEE analysis in the present study shows a 0.226 mm increase in axial length during a 1% decrease in hemoglobin glycate. Utaal et al. observed in their study that the axial length of the eyeball decreased with the progression of diabetic retinopathy [25]. Also, Huntjens et al., in their study, state that the axial length in the group with uncontrolled blood sugar is less than the control group, [24] and the results of both studies confirm the results of the GEE analysis of the present study.
In their study, Ye et al. observed that the power of crystalline lenses in people with type 2 diabetes was higher than in normal individuals, [19] so that the reduction of the central refractive index of the crystalline lens is an influential factor in the hyperopic shift created in the process of reducing the blood sugar [29].
Therefore, the reason for the lack of change in the refraction in the findings of this study, despite the change in axial length, which is one of the most critical components determining refractive status, can be interpreted as reducing the crystalline strength of the lens in the process of hypoglycemia, neutralizing the effect of increasing axial length. Therefore, the result did not change the eye refraction. A 1% reduction in the amount of hemoglobin glycate cannot change the lens crystal’s central refractive index and thus the eye’s refractive error.
Conclusion
The present study shows that the refractive and biometric parameters of the eye at one and a half and three months after the start of diabetes treatment do not show a significant difference compared to the baseline. Therefore, the clinical point that can be deduced from this study is that in patients who started their diabetes treatment more than a month and a half ago, it is not required to delay decision-making about the refraction and biometric parameters of the eye, and the necessary examinations can be performed, of course when the pre-treatment hemoglobin glycate was around 7.5 %.
Ethical Considerations
Compliance with ethical guidelines
This study was reviewed and approved by the Ethics Committee of Iran University of Medical Sciences (Code: IR.IUMS.REC.1399.418).
Funding
The paper was extracted from the PhD dissertation of the Sattar Rajabi entitled "Evaluation of Change of anterior segment properties and refractive characteristics of the eye in patients with type 2 hyperglycemia before and after therapy and determination of their relationship with changes in HbA1c values" that was presented at the Iran University of Medical Sciences 2021.
Authors' contributions
All authors contributed to the data analysis, revision of the article, and final approval of the version to be published, and agree to be accountable for all aspects of the work.
Conflict of interest
There is no conflict of interests for any authors.
Acknowledgments
The authors thank the participants who made this study possible.
References
According to the International Diabetes Federation in 2017, 451 million people worldwide had diabetes, and this number is projected to reach 693 million by 2045 [1]. Diabetes is the most common endocrine disease in countries; [2] and hyperglycemia is an essential feature, and a risk factor for its formation; [3] hemoglobin glycate is the most valid laboratory test to assess blood sugar [4]. Acute and chronic changes in blood sugar cause changes in the structure of the eye, such as fluctuations in near and far vision and ocular biometric parameters [5, 6]. It is essential to be aware of the current situation and predict possible changes in the individual’s treatment path in making decisions about the possibility of making changes in the individual’s optical correction.
Various studies have been performed to evaluate the refractive error in people with diabetes compared to normal people [5, 7, 8] and its changes after being in the process of hypoglycemia [9-11] so that the common finding of them is Myopia shift in people with uncontrolled blood sugar [12] and hyperopic shift after being in the process of hypoglycemia [10]. However, sometimes the opposite results have been presented in studies; among them, it is possible to mention the slight shift of hyperopia after transient hyperglycemia, [13] no change in refraction during blood sugar fluctuation, [14, 15] and myopic shift during hyperglycemia reduction [16].
A set of biometric factors, such as axial length, corneal power, and crystalline thickness of the lens, along with the refractive index of the cornea, lens, aqueous humor, and vitreous, determine the refractive error of the eye, [17] therefore any change in these parameters due to reduced blood sugar may cause changes in the refractive error changes in the eyes of people with diabetes. Among the most critical changes in biometric parameters while changes in blood sugar are changes in the structure and dimensions of the crystalline lens, [18] whose related changes in blood sugar are controversial [19]. Axial length is also considered to be an essential component associated with refractive error changes and diabetic retinopathy [20].
In the present study, while examining a group of people with hemoglobin glycate of more than 7.5%, the changes in biometric parameters, the components of refractive error, and the relationship between them with blood sugar reduction are evaluated.
Materials and Methods
Participants
The present study was conducted at the Iran University of Medical Sciences in 2020. People with the first diagnosis of type 2 diabetes or a history of poor blood sugar control were evaluated. In addition, the inclusion criteria included having hemoglobin glycate of more than 7.5% and the absence of any ocular and systemic diseases, including abnormal retinal and corneal manifestations, eye pressure of more than 21 mm Hg, hypertension, hyperlipidemia, anemia, history of any eye surgery, and long-term use of systemic and topical steroids.
Examinations
People with hemoglobin glycate of more than seven and a half were referred to the optometry department for evaluation and eye examinations by an internal medicine specialist. Eye examinations of the anterior and posterior segments were performed using a slit lamp (Haag-Streit corp., Switzerland) with a 90-diopter Volk lens to evaluate the presence criteria.
For patients who met the inclusion criteria, refraction and biometrics were recorded from both eyes. First, uncorrected visual acuity was recorded by the Snllen chart with logMAR criteria. Then, objective refraction was performed by Tono ref 2 (Autoref/Kerato/Tonometer) Nidek, Japan, and the results were confirmed by a retinoscope (Heine Beta 200, Heine Optotechnik, Germany). It should be noted that spherical components, spherical equivalents, and J0 and J45 parameters were recorded based on objective values. In the next step, ocular biometric evaluation was performed by IOLMaster 700 (Carl Zeiss Meditec, Jena, Germany), and the parameters of axial length, anterior chamber depth, and crystalline lens thickness were measured, and biometric and refraction data sets were analyzed.
The process of treatment and reduction of blood sugar was started under the supervision of an internal medicine specialist, and all the above measurements were recorded along with hemoglobin glycated level after one and a half and three months from the beginning of the process of treatment of hypoglycemia; finally, statistical analysis was performed on the findings after recording all the information. It should be noted that all measurements in each examination were performed on the same day as the measurement of hemoglobin glycate.
Statistical analysis
The present study presents descriptive information, including Mean±SD, and 95% confidence interval for all parameters.
Vector analysis to examine the change in astigmatism considering (S [sphere], C [cylinder], α [axis]) and calculating J0=-(C/2)×cos(2α), J45=-(C/2) sin(2α) and SE=S+C/2 were performed [20].
Analytical analysis was performed after examining the normality of data distribution by the Kolmogorov-Smirnov test. The mean differences of each parameter in the three examinations were checked by repeated-measures analysis of variance (ANOVA), and if the difference was significant, pair-wise differences were evaluated by Bonferroni correction. Due to the correlation of data between the two eyes, to analyze the relationship between changes in each parameter while changes in blood sugar between the three examinations during blood sugar control were examined by generalized estimating equation (GEE) analysis. It should be noted that all statistical analyzes were performed by SPSS software, version 25 (IBM corporation, Armonk, N.Y.) at a significant level of 5%.
Results
In the present study, 60 eyes of 30 patients with a Mean±SD age of 51.63±6.79 years (25 to 60 years) were evaluated so that the number of people in both gender groups was equal.
Based on the information in Table 1, which shows the amount of hemoglobin glycate measured in each examination, it can be seen that this parameter has decreased by an average of 1.028% compared to the baseline measurement in the third month (P<0.001).
.png)
The process of lowering blood sugar was such that hemoglobin glycated levels were different from each other in the first and second examinations (P<0.001), first and third (P<0.001), and second and third (P=0.003). It should be mentioned in tables FU1means Follow-up 1.5 months and FU2 means Follow-up 3 months.
ANOVA with repeated measures showed no difference between spherical and spherical mean in three examinations (all, P>0.05) so that the spherical equivalent in the three examinations was -0.20±-1.55, -0.25±-1.55, and -0.25±-1.54 diopters, respectively (P=0.340). Also, despite the difference in the numerical value of the parameters J0 (P=0.143) and J45 (P=0.910) in each examination, this discrepancy was not statistically significant (P>0.05), Table 2 presents these data.

Among the studied biometric parameters (Table 2), only the average thickness of the crystalline lens was different between the three examinations (P=0.001) so that pair-wise evaluation showed the difference in the crystalline lens thickness between the first and third examinations (P=0.009, MD=+0.52 mm) and between the second and third examination (P=0.005, MD=+0.64 mm). The evaluation of mean values of axial length parameters (P=0.147) and anterior chamber depth (P=0.336) did not differ significantly between the three evaluations.
Finally, GEE analysis showed that among all the studied parameters (Table 3), the axial length of the eyeball changed while lowering blood sugar.

In fact, despite the lack of difference in the mean axial length in the three examinations (P=0.147), a 1% decrease in hemoglobin glycate increased the axial length by 0.226 mm (P=0.002); GEE analysis showed no significant relationship between changes in other parameters, including refractive error components, lens thickness, and anterior chamber depth with glycemic control (all, P>0.05).
Discussion
Being on the path of lowering blood sugar is the crucial action after the diagnosis of diabetes, which is one of the unwanted side effects in some people; [10] therefore it is recommended to avoid any optical prescription in conditions where the blood sugar level is abnormally high or low [12]. Now the answer to how long to wait after treatment is to record the stability of refractive changes is clinically significant.
The present study results show no change in the spherical, cylindrical, and spherical equivalent and a 1% decrease in hemoglobin glycate within three months after the start of diabetic treatment. The exact mechanism of refractive error changes in blood glucose changes has not yet been elucidated well. However, some previous studies have shown that severe and sudden glycemic decrease leads to hyperopic shifts in refraction [9, 11]. Peak shift in hyperopia has been observed in different studies on the seventh day (Li et al.), [21] tenth day (Okamoto et al.), [10] between 7 to 14 days (Saito et al.), [22] and finally on the seventeenth day (Lin et al.) [9] after starting treatment.
The hyperopic shift created in the process of glycemic control gradually decreases, and returning to baseline level has also been mentioned in different studies, such as in the study of Li et al. This time was in two to four weeks after starting treatment [21] and Okamoto et al.’ study reported 14 to 84 days after the initial evaluation. [10].
The set of results of the mentioned studies along with the results of the present study can be interpreted as one of the reasons for the lack of difference in refractive error values observed between the baseline and the first examination, due to the time difference of one and a half months between the two examinations. Therefore, the created hyperopic shift will be expected to return to the base level from this period. It has also been shown a direct relationship between the rate of maximal hyperopic shift and the timing of peak changes since the start of glycemic control treatment [10].
On the other hand, the blood sugar level of the evaluated individuals also affected the observed results so that Ebeigbe found that the transient refractive error changes in newly diagnosed diabetic patients depended on blood glucose levels [23]. The mean of hemoglobin glycate in the pre-treatment evaluation and the maximum changes in refraction in the study of Li et al. was 12.20±1.50% and 1.6 diopters of hyperopic shift, respectively [21]. In Okamoto et al., it was equal to 11.90±2.8% and 1.47 diopters of hyperopic shift [10] and in the present study, it was equal to 8.82±1.31% without refractive error changes. According to the above, it can be concluded that the lower blood sugar level of the subjects in the present study compared to other studies is also one of the compelling reasons for not observing changes in refractive error in the examination before and after the person’s presence in the process of reduction of blood sugar.
Therefore, it is predictable that even if you do not know the status of the refractive error line, you can know the amount of hemoglobin glycate in the person before starting treatment for hypoglycemia, the number of days elapsed since the start of treatment, and the amount of decreased blood glucose. Thus, we gained valuable information about the individual’s condition and decided on an optical prescription based on pre-treatment data.
Evaluation of biometric parameters in the present study showed that the average crystal lens thickness in the third examination is lower than the first and the third examination than the second. However, the results of GEE analysis show that the differences observed in this parameter cannot be directly attributed to the changes in blood sugar. Decreasing the crystalline lens thickness in the process of lowering blood sugar is a common finding that has been mentioned in various studies [10, 19]. Besides, Huntjens et al. observed that a 2% short-term increase in glycated hemoglobin caused a 0.21 mm increase in the crystalline thickness of the lens [24]. However, in some previous studies, differences in this parameter in the treatment process of diabetes [11] and also in the comparison of people with type 2 diabetes and normal people [15] were not significant; it may be inferred that the rate of change in hemoglobin glycated was the cause of the observed difference in crystalline lens thickness changes in the process of glycemic control.
Also, the results of the present study showed no change in the average anterior chamber depth while reducing blood sugar. Lack of difference in the depth of the anterior chamber of the eyeball while lowering blood sugar is a common finding reported in various studies. [10, 25] However, in their study, Kocaturk et al. found that the average anterior chamber depth in the right eye of people with uncontrolled type 2 diabetes was lower than in normal people; this difference was not observed in the left eye of the same individuals [26].
It is commonly accepted that reducing the crystalline thickness of the lens increases the depth of the anterior chamber of the eyeball [27]. Therefore, it is expected that the average thickness of the crystalline lens in the third month after the start of treatment compared to the baseline was associated with a greater depth of the anterior chamber. Two possible reasons exist for this, the first reason is that changes in corneal curvature may have occurred during treatment, neutralizing the effect of changes in lens thickness on anterior chamber depth, or that the convexity of the lens may have shifted further toward the vitreous, which has not affected the depth of the anterior chamber [28].
The results of the present study show no difference in the mean axial length of the eyeball measured between the three examinations. This finding was similarly observed in the studies of Li et al. and Seven et al. [11, 21] However, GEE analysis in the present study shows a 0.226 mm increase in axial length during a 1% decrease in hemoglobin glycate. Utaal et al. observed in their study that the axial length of the eyeball decreased with the progression of diabetic retinopathy [25]. Also, Huntjens et al., in their study, state that the axial length in the group with uncontrolled blood sugar is less than the control group, [24] and the results of both studies confirm the results of the GEE analysis of the present study.
In their study, Ye et al. observed that the power of crystalline lenses in people with type 2 diabetes was higher than in normal individuals, [19] so that the reduction of the central refractive index of the crystalline lens is an influential factor in the hyperopic shift created in the process of reducing the blood sugar [29].
Therefore, the reason for the lack of change in the refraction in the findings of this study, despite the change in axial length, which is one of the most critical components determining refractive status, can be interpreted as reducing the crystalline strength of the lens in the process of hypoglycemia, neutralizing the effect of increasing axial length. Therefore, the result did not change the eye refraction. A 1% reduction in the amount of hemoglobin glycate cannot change the lens crystal’s central refractive index and thus the eye’s refractive error.
Conclusion
The present study shows that the refractive and biometric parameters of the eye at one and a half and three months after the start of diabetes treatment do not show a significant difference compared to the baseline. Therefore, the clinical point that can be deduced from this study is that in patients who started their diabetes treatment more than a month and a half ago, it is not required to delay decision-making about the refraction and biometric parameters of the eye, and the necessary examinations can be performed, of course when the pre-treatment hemoglobin glycate was around 7.5 %.
Ethical Considerations
Compliance with ethical guidelines
This study was reviewed and approved by the Ethics Committee of Iran University of Medical Sciences (Code: IR.IUMS.REC.1399.418).
Funding
The paper was extracted from the PhD dissertation of the Sattar Rajabi entitled "Evaluation of Change of anterior segment properties and refractive characteristics of the eye in patients with type 2 hyperglycemia before and after therapy and determination of their relationship with changes in HbA1c values" that was presented at the Iran University of Medical Sciences 2021.
Authors' contributions
All authors contributed to the data analysis, revision of the article, and final approval of the version to be published, and agree to be accountable for all aspects of the work.
Conflict of interest
There is no conflict of interests for any authors.
Acknowledgments
The authors thank the participants who made this study possible.
References
- Pheiffer C, Pillay-van Wyk V, Joubert JD, Levitt N, Nglazi MD, Bradshaw D. The prevalence of type 2 diabetes in South Africa: A systematic review protocol. BMJ Open. 2018; 8(7):e021029-e. [DOI:10.1136/bmjopen-2017-021029] [PMID] [PMCID]
- Kaštelan S, Gverović-Antunica A, Pelčić G, Gotovac M, Marković I, Kasun B. Refractive changes associated with diabetes mellitus. Semi in Ophthalmol. 2018; 33(7):838-45. [DOI:10.1080/08820538.2018.1519582] [PMID]
- Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al.Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012; 35(3):556-64. [DOI:10.2337/dc11-1909] [PMID] [PMCID]
- Saudek CD, Brick JC. The clinical use of hemoglobin A1c. Journal of Diabetes Science and Technology. 2009; 3(4):629-34. [DOI:10.1177/193229680900300402] [PMID] [PMCID]
- Wiemer NG, Eekhoff EM, Simsek S, Heine RJ, Ringens PJ, Polak BC, et al. Refractive properties of the healthy human eye during acute hyperglycemia. Journal of Clinical and Experimental Ophthalmology. 2008; 246(7):993-8. [DOI:10.1007/s00417-008-0810-y] [PMID] [PMCID]
- Xiao Y, Li T, Jia Y, Wang S, Yang C, Zou H. Influence of type 1 diabetes mellitus on the ocular biometry of Chinese children. Journal of Ophthalmol. 2019; 2019:7216490. [DOI:10.1155/2019/7216490] [PMID] [PMCID]
- Klein BEK, Lee KE, Klein R. Refraction in adults with diabetes. Archives of Ophthalmol. 2011; 129(1):56-62. [DOI:10.1001/archophthalmol.2010.322] [PMID] [PMCID]
- Song E, Qian D-J, Wang S, Xu C, Pan C-W. Refractive error in Chinese with type 2 diabetes and its association with glycaemic control. Clinical and Experimental Optometry. 2018; 101(2):213-9. [DOI:10.1111/cxo.12606] [PMID]
- Lin SF, Lin PK, Chang FL, Tsai RK. Transient hyperopia after intensive treatment of hyperglycemia in newly diagnosed diabetes. Ophthalmologica. 2009; 223(1):68-71. [DOI:10.1159/000173714] [PMID]
- Okamoto F, Sone H, Nonoyama T, Hommura S. Refractive changes in diabetic patients during intensive glycaemic control. British Journal of Ophthalmol. 2000; 84(10)1097. [DOI:10.1136/bjo.84.10.1097] [PMID] [PMCID]
- Seven E, Yıldız S, Tekin S, Altaş AS, Özer MD, Batur M, et al. Effect of Insulin Therapy on Ocular Biometric Parameters in Diabetic Patients. Journal of Ocular Pharmacology and Therapeutics. 2020; 36(2):102-8. [DOI:10.1089/jop.2019.0070] [PMID]
- Majola L, Munsamy AJ. A review of glycaemic changes on vision in phakic, aphakic and pseudophakic people with diabetes. African Vision and Eye Health. 2020; 79(1):1-9. [DOI:10.4102/aveh.v79i1.509]
- Tai MC, Lin SY, Chen JT, Liang CM, Chou PI, Lu DW. Sweet hyperopia: Refractive changes in acute hyperglycemia. European Journal of Ophthalmology. 2006; 16(5):663-6. [DOI:10.1177/112067210601600501] [PMID]
- Agardh E, Hellgren KJ, Bengtsson B. Stable refraction and visual acuity in diabetic patients with variable glucose levels under routine care. Acta Ophthalmologica. 2011; 89(2):107-10. [DOI:10.1111/j.1755-3768.2009.01664.x] [PMID]
- Wiemer NG, Dubbelman M, Ringens PJ, Polak BC. Measuring the refractive properties of the diabetic eye during blurred vision and hyperglycaemia using aberrometry and Scheimpflug imaging. Acta Ophthalmologica. 2009; 87(2):176-82. [DOI:10.1111/j.1755-3768.2008.01212.x] [PMID]
- Yarbağ A, Yazar H, Akdoğan M, Pekgör A, Kaleli S. Refractive errors in patients with newly diagnosed diabetes mellitus. Pakistan Journal of Medical Sciences. 2015; 31(6):1481-4. [DOI:10.12669/pjms.316.8204] [PMID] [PMCID]
- Perkins ES. Cataract: Refractive error, diabetes, and morphology. British Journal of Ophthalmology. 1984; 68(5):293-7. [DOI:10.1136/bjo.68.5.293] [PMID] [PMCID]
- Mehta VV, Hull CC, Lawrenson JG. The effect of varying glucose levels on the ex vivo crystalline lens: Implications for hyperglycaemia-induced refractive changes. Ophthalmic and Physiological Optics. 2015; 35(1):52-9. [DOI:10.1111/opo.12176] [PMID]
- Ye L, He J, Zhang X, Xu Y, Chen Q, Yin Y, et al. The associations of lens power with age, axial length and type 2 diabetes mellitus in Chinese adults aged 50 and above. Eye and Vision. 2020;77(1):57. [DOI:10.1186/s40662-020-00222-2] [PMID] [PMCID]
- Meng W, Butterworth J, Malecaze F, Calvas P. Axial length of myopia: A review of current research. Ophthalmologica. 2011; 225(3):127-34. [DOI:10.1159/000317072] [PMID]
- Li HY, Luo GC, Guo J, Liang Z. Effects of glycemic control on refraction in diabetic patients. International Journal of Ophthalmology. 2010; 3(2):158-60. [PMCID] [PMID]
- Saito Y, Ohmi G, Kinoshita S, Nakamura Y, Ogawa K, Harino S, et al. Transient hyperopia with lens swelling at initial therapy in diabetes. British Journal of Ophthalmology. 1993; 77(3):145-8. [DOI:10.1136/bjo.77.3.145] [PMID] [PMCID]
- JA Ebeigbe, AB. Osaiyuwu. Transient refractive changes in a newly diagnose diabetic-a case report. Journal of the Nigerian Optometric Association. 2009; 15(1):28-32. [DOI:10.4314/jnoa.v15i1.55607]
- Huntjens B, Charman WN, Workman H, Hosking SL, O’Donnell C. Short-term stability in refractive status despite large fluctuations in glucose levels in diabetes mellitus type 1 and 2. PloS One. 2012; 7(12):e52947. [DOI:10.1371/journal.pone.0052947] [PMID] [PMCID]
- Utaal SKD, Chopra R, Batra N. Association of ocular biometric parameters with diabetic retinopathy. International Journal of Research in Medical Sciences. 2020; 8(10):3624-8. [DOI:10.18203/2320-6012.ijrms20204240]
- Kocatürk T, Zengin M, Cakmak H, Evliçoglu GE, Dündar SO, Omürlü IK, et al. The ocular biometric differences of diabetic patients. European Journal of Ophthalmol. 2014; 24(5):786-9. [DOI:10.5301/ejo.5000446] [PMID]
- Jamali A, Naghdi T, Abardeh MH, Jamalzehi M, Khalajzadeh M, Kamangar M, et al. Ocular biometry characteristics in cataract surgery candidates: A cross-sectional study. MEHDI in Ophthalmol. 2021; 10(1):11-7. [DOI:10.51329/mehdiophthal1416]
- Hashemi H, Yekta A, Yazdani N, Ostadimoghaddam H, Khabazkhoob M. Comparison of anterior chamber depth between normal and keratoconic eyes: A systematic review and meta-analysis. Journal of Current Ophthalmol. 2020; 3(1):94-8. [DOI:10.1016/j.joco.2019.01.010]
- Charman WN, Adnan, Atchison DA. Gradients of refractive index in the crystalline lens and transient changes in refraction among patients with diabetes. Biomedical Optics Express. 2012; 3(12):3033-42. [Link]
Type of Study: Research |
Subject:
Optometry
Received: 2022/12/13 | Accepted: 2023/02/13 | Published: 2022/02/3
Received: 2022/12/13 | Accepted: 2023/02/13 | Published: 2022/02/3





